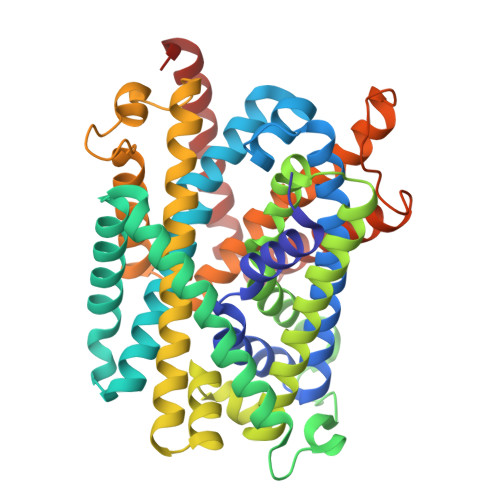
The conserved lysine residue in transmembrane helix 5 is pivotal for the cytoplasmic gating of the L-amino acid transporters.
Fort, J., Nicolas-Arago, A., Maggi, L., Martinez-Molledo, M., Kapiki, D., Gonzalez-Novoa, P., Gomez-Gejo, P., Zijlstra, N., Bodoy, S., Pardon, E., Steyaert, J., Llorca, O., Orozco, M., Cordes, T., Palacin, M.(2025) PNAS Nexus 4: pgae584-pgae584
- PubMed: 39822574
- DOI: https://doi.org/10.1093/pnasnexus/pgae584
- Primary Citation of Related Structures:
9H76 - PubMed Abstract:
L-Amino acid transporters (LATs) play a key role in a wide range of physiological processes. Defects in LATs can lead to neurological disorders and aminoacidurias, while the overexpression of these transporters is related to cancer. BasC is a bacterial LAT transporter with an APC fold. In this study, to monitor the cytoplasmic motion of BasC, we developed a single-molecule Förster resonance energy transfer assay that can characterize the conformational states of the intracellular gate in solution at room temperature. Based on combined biochemical and biophysical data and molecular dynamics simulations, we propose a model in which the conserved lysine residue in TM5 supports TM1a to explore both open and closed states within the cytoplasmic gate under apo conditions. This equilibrium can be altered by substrates, mutation of conserved lysine 154 in TM5, or a transport-blocking nanobody interacting with TM1a. Overall, these findings provide insights into the transport mechanism of BasC and highlight the significance of the lysine residue in TM5 in the cytoplasmic gating of LATs.
- Institute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technology, Baldiri Reixac 10, 08028 Barcelona, Spain.
Organizational Affiliation: