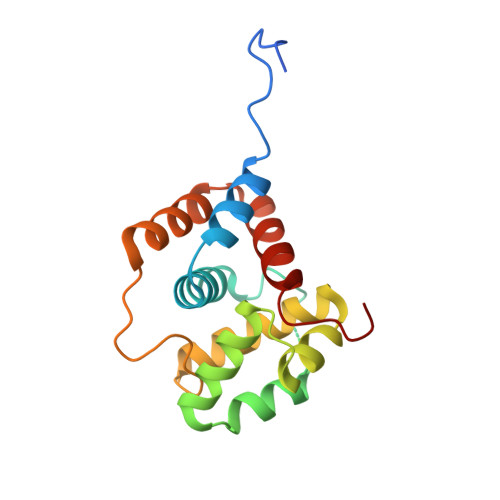
The apo-HCP4 structure reveals facets of carotenoid uptake.
Sklyar, J., Glaser, F., Adir, N.(2025) Int J Biol Macromol 315: 144290-144290
- PubMed: 40398757
- DOI: https://doi.org/10.1016/j.ijbiomac.2025.144290
- Primary Citation of Related Structures:
9H41 - PubMed Abstract:
Photosynthetic organisms employ sophisticated mechanisms to mitigate photodamage caused by excessive light energy. Among these, proteins such as the Orange Carotenoid Protein (OCP) and the Helical Carotenoid Protein 4 (HCP4) play a central role in non-photochemical quenching (NPQ), by dissipating excess energy. OCP consists of two domains: the N-terminal domain (NTD), which serves as the effector domain, and the C-terminal domain (CTD), which acts as the regulatory domain. The HCPs, which are homologs of the NTD, perform carotenoid-driven energy quenching, carotenoid transport and reactive oxygen species scavenging in cyanobacteria. CTD homologs (CTDH) are involved in carotenoid uptake and delivery. This study presents the first crystal structure of an apo-HCP4 from Anabaena sp. PCC 7120 at a resolution of 3.1 Å, revealing significant structural differences from the previously determined carotenoid bound form (holo-HCP4). The ligand-binding cavity in apo-HCP4 is occluded by dynamic loops, that must move to afford carotenoid transfer from a transiently bound holo-CTDH. The predicted conformational changes of HCP4 loops and the mobility of CTDH residues create a favorable environment for efficient ligand transfer. Our structural analysis identified HCP4 Tyr48 as a key gating residue, regulating cavity accessibility during carotenoid uptake. Biolayer interferometry experiments demonstrated that apo-HCP4 requires interaction with holo-CTDH for effective carotenoid transfer, emphasizing the interplay between these proteins in the carotenoid transport mechanism. Our findings provide insights into the structural and functional differences between apo- and holo-HCP4, elucidating the regulatory mechanisms underlying carotenoid transport and energy quenching in cyanobacteria. This study advances our understanding of carotenoid-mediated photoprotection and transport.
- Schulich Faculty of Chemistry, Technion, Haifa 3200003, Israel.
Organizational Affiliation: