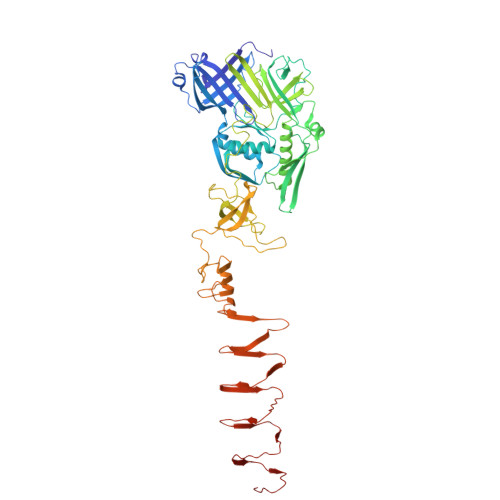
Function and firing of the Streptomyces coelicolor contractile injection system requires the membrane protein CisA.
Casu, B., Sallmen, J.W., Haas, P.E., Chandra, G., Afanasyev, P., Xu, J., Pilhofer, M., Schlimpert, S.(2025) Elife 14
- PubMed: 40626860
- DOI: https://doi.org/10.7554/eLife.104064
- Primary Citation of Related Structures:
9GTP, 9GTR, 9GTS - PubMed Abstract:
Bacterial contractile injection systems (CIS) are phage tail-like macromolecular complexes that mediate cell-cell interactions by injecting effector proteins into target cells. CIS from Streptomyces coelicolor (CIS Sc ) are localized in the cytoplasm. Under stress, they induce cell death and impact the Streptomyces life cycle. It remains unknown, however, whether CIS Sc require accessory proteins to directly interact with the cytoplasmic membrane to function. Here, we characterize the putative membrane adaptor CisA, a conserved factor in CIS gene clusters across Streptomyces species. We show by cryo-electron tomography imaging and in vivo assays that CIS Sc contraction and function depend on CisA. Using single-particle cryo-electron microscopy, we provide an atomic model of the extended CIS Sc apparatus; however, CisA is not part of the complex. Instead, our findings show that CisA is a membrane protein with a cytoplasmic N-terminus predicted to interact with CIS Sc components, thereby providing a possible mechanism for mediating CIS Sc recruitment to the membrane and subsequent firing. Our work shows that CIS function in multicellular bacteria is distinct from type VI secretion systems and extracellular CIS, and possibly evolved due to the role CIS Sc play in regulated cell death.
- Department of Biology, Institute of Molecular Biology & Biophysics, Eidgenössische Technische Hochschule Zürich, Zurich, Switzerland.
Organizational Affiliation: