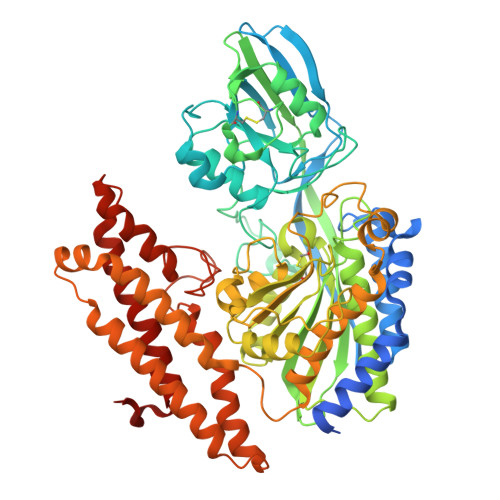
Conjugation to a transferrin receptor 1-binding Bicycle peptide enhances ASO and siRNA potency in skeletal and cardiac muscles.
Ostergaard, M.E., Carrer, M., Anderson, B.A., Afetian, M., Bakooshli, M.A., Santos, J.A., Klein, S.K., Capitanio, J., Freestone, G.C., Tanowitz, M., Galindo-Murillo, R., Gaus, H.J., Dwyer, C.A., Jackson, M., Jafar-Nejad, P., Rigo, F., Seth, P.P., Gaynor, K.U., Stanway, S.J., Urbonas, L., St Denis, M.A., Pellegrino, S., Bezerra, G.A., Rigby, M., Gowans, E., Van Rietschoten, K., Beswick, P., Chen, L., Skynner, M.J., Swayze, E.E.(2025) Nucleic Acids Res 53
- PubMed: 40207629
- DOI: https://doi.org/10.1093/nar/gkaf270
- Primary Citation of Related Structures:
9GH7 - PubMed Abstract:
Improving the delivery of antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs) to skeletal and cardiac muscles remains a pivotal task toward the broader application of oligonucleotide therapeutics. The targeting of myofibers and cardiomyocytes via conjugation of ASOs and siRNAs to ligands that bind the human transferrin receptor 1 (TfR1) has gathered significant interest in recent years. However, the selection of ligands with low molecular weight and optimal biophysical and binding properties is crucial to maximize the potential of the TfR1 ligand-conjugated antisense (LICA) technology. Here, through effective combination of phage display and peptide medicinal chemistry, we identified and characterized a bicyclic peptide (Bicycle® molecule BCY17901), with a molecular weight of ∼2 kDa, that binds human TfR1 with high affinity and specificity. Conjugation to BCY17901 improved ASO and siRNA potency in skeletal and cardiac muscles of human TfR1 knock-in mice, after either intravenous or subcutaneous administration. Furthermore, single-nucleus RNA sequencing showed that conjugation to BCY17901 enhanced ASO activity in myonuclei of different muscle fiber types. Importantly, we demonstrated good translatability of our TfR1-targeting platform in skeletal and cardiac muscles of nonhuman primates. Our results offer great promise toward potential future applications of low-molecular-weight Bicycle LICA therapeutics for the treatment of diseases affecting skeletal muscle and heart.
- Ionis Pharmaceuticals Inc., 2855 Gazelle Court, Carlsbad, CA 92010, United States.
Organizational Affiliation: