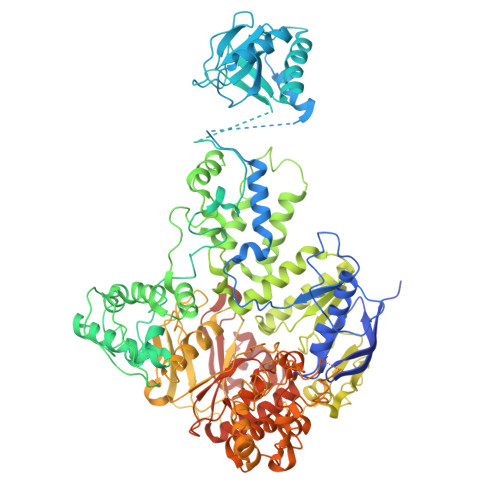
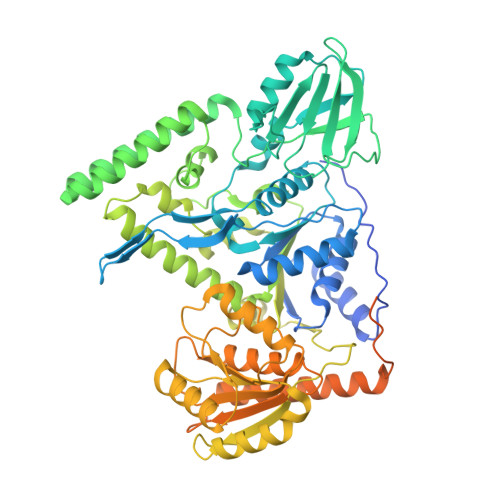
Mechanistic understanding of UvrA damage detection and lesion hand-off to UvrB in Nucleotide Excision Repair.
Genta, M., Ferrara, G., Capelli, R., Rondelli, D., Sertic, S., Bolognesi, M., Rizzi, M., Rossi, F., Jeruzalmi, D., Chaves-Sanjuan, A., Miggiano, R.(2025) Nat Commun 16: 3416-3416
- PubMed: 40210888
- DOI: https://doi.org/10.1038/s41467-025-58670-0
- Primary Citation of Related Structures:
9GA2, 9GA3, 9GA4, 9GA5 - PubMed Abstract:
Nucleotide excision repair (NER) represents one of the major molecular machineries that control chromosome stability in all living species. In Eubacteria, the initial stages of the repair process are carried out by the UvrABC excinuclease complex. Despite the wealth of structural data available, some crucial details of the pathway remain elusive. In this study, we present a structural investigation of the Mycobacterium tuberculosis UvrAUvrB complex and of the UvrA dimer, both in complex with damaged DNA. Our analyses yield insights into the DNA binding mode of UvrA, showing an unexplored conformation of Insertion Domains (IDs), underlying the essential role of these domains in DNA coordination. Furthermore, we observe an interplay between the ID and the UvrB Binding Domain (UBD): after the recognition of the damage, the IDs repositions with the concomitant reorganization of UBD, allowing the formation of the complex between UvrA and UvrB. These events are detected along the formation of the uncharacterized UvrA 2 UvrB 1 -DNA and the UvrA 2 UvrB 2 -DNA complexes which we interpret as hierarchical steps initiating the DNA repair cascade in the NER pathway, resulting in the formation of the pre-incision complex.
- Department of Pharmaceutical Sciences, University of Piemonte Orientale, Via Bovio 6, 28100, Novara, Italy.
Organizational Affiliation: