Crystal structure of mouse Ces2e
Eisner, H., Rammer, L., Riegler-Berket, L., Rodriguez Gamez, C., Sagmeister, T., Chalhoub, G., Liesinger, L., Birner-Gruenberger, R., Pavkov-Keller, T., Haemmerle, G., Schoiswohl, G., Oberer, M.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Pyrethroid hydrolase Ces2e | 565 | Mus musculus | Mutation(s): 0 Gene Names: Ces2e, Ces5 EC: 3.1.1.88 (PDB Primary Data), 3.1.1 (PDB Primary Data), 3.1.1.28 (UniProt) | 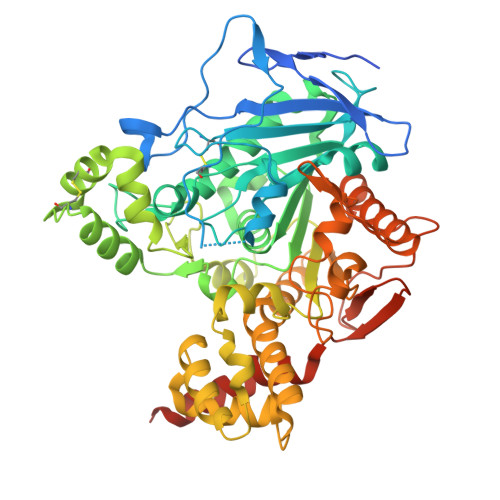 | |
UniProt | |||||
Find proteins for Q91WG0 (Mus musculus) Explore Q91WG0 Go to UniProtKB: Q91WG0 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q91WG0 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | Go to GlyGen: Q91WG0-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose | C | 6 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G82348BZ GlyCosmos: G82348BZ GlyGen: G82348BZ | |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-2)-alpha-D-mannopyranose-(1-3)-[2-acetamido-2-deoxy-beta-D-glucopyranose-(1-2)-alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose | D | 8 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G80858MF GlyCosmos: G80858MF GlyGen: G80858MF | |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | E [auth A] F [auth A] G [auth A] H [auth A] I [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| CL Query on CL | L [auth A], M [auth A], V [auth B], W [auth B] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 126.931 | α = 90 |
| b = 145.243 | β = 90 |
| c = 149.432 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Austrian Science Fund | Austria | DOI: 10.55776/F73 |
| Austrian Science Fund | Austria | DOI: 10.55776/DOC50 |