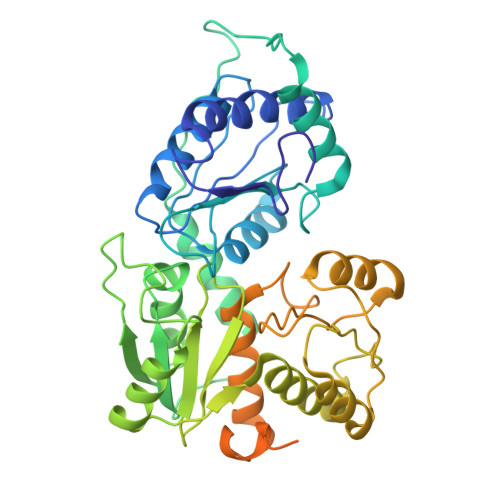
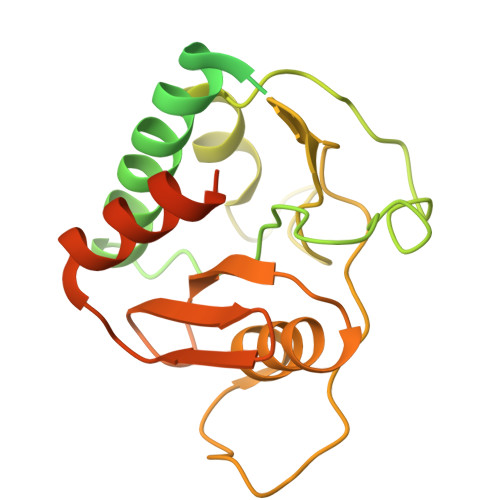
Membrane-embedded CdaA is required for efficient synthesis of second messenger cyclic di-AMP.
Foster, A.J., Li, H., Drougkas, P., Schuurman-Wolters, G.K., Ten Kate, J., Paulino, C., Poolman, B.(2024) Commun Biol 7: 1710-1710
- PubMed: 39739009
- DOI: https://doi.org/10.1038/s42003-024-07420-x
- Primary Citation of Related Structures:
9G69 - PubMed Abstract:
Cyclic di-adenylate monophosphate (cyclic di-AMP) is an important second messenger in microorganisms. Cyclic di-AMP regulates bacterial cell volume and turgor via control of potassium and compatible solute transport but is also involved in many other processes, including the activation of the metazoan innate immune response to bacterial infections. We compare the activity of full-length membrane-embedded CdaA, the enzyme that synthesizes cyclic di-AMP, with the water-soluble catalytic domain CdaA-DAC. Purified CdaA from L. lactis was studied in the detergent-solubilized state, and in lipid nanodiscs and vesicles. We show that CdaA is tetrameric and the membrane-bound complex has more than 2-orders of magnitude higher activity than soluble CdaA-DAC. CdaA activity increases with pH but does not strongly depend on the salt or lipid content, factors that are crucial for the control of osmoregulatory transporters. Cryo-EM and in-silico structure prediction of CdaA show that the two DAC dimers engage in a head-to-head interaction, leading to cyclic-di-AMP formation. The inhibitor phosphoglucomutase prevents this active conformation. We observe dynamic flexibility between the catalytic and membrane domains, even in the presence of ATP or non-hydrolyzable substrate ApCpp. This is the first comprehensive functional and structural characterization of a full-length cyclic di-AMP-specific cyclase.
- Department of Biochemistry, Groningen Biomolecular Science and Biotechnology Institute, University of Groningen, Nijenborgh 3, Groningen, The Netherlands.
Organizational Affiliation: