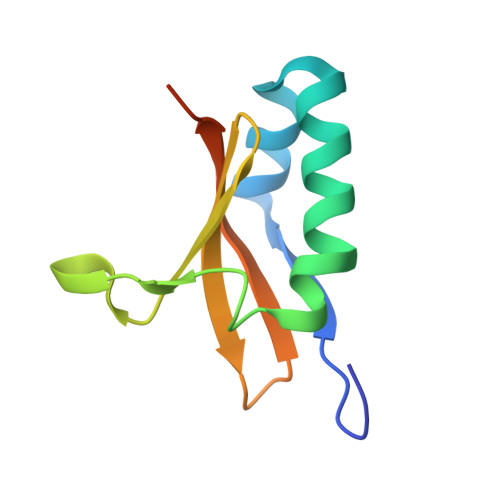
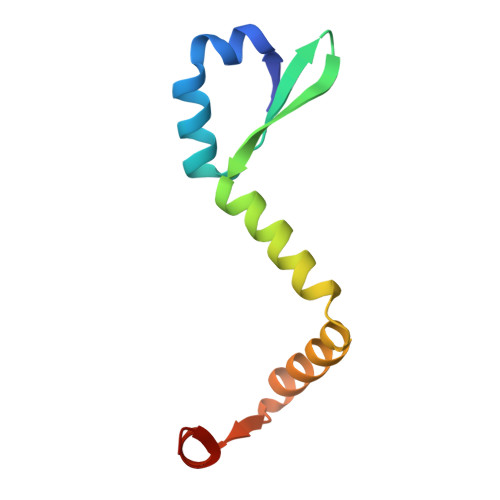
Ribonuclease toxin RelE1 inhibits growth of Mycobacterium tuberculosis through specific cleavage of the ribosomal anti-Shine-Dalgarno region.
Han, X., Beck, I.N., Mansour, M., Arrowsmith, T.J., Barriot, R., Chansigaud, P., Pages, C., Hamze, H., Akarsu, H., Falquet, L., Redder, P., Xu, X., Blower, T.R., Genevaux, P.(2025) Nucleic Acids Res 53
- PubMed: 41242526
- DOI: https://doi.org/10.1093/nar/gkaf1070
- Primary Citation of Related Structures:
9G12 - PubMed Abstract:
Toxin-antitoxin (TA) systems are central to bacterial immunity, genome maintenance, and pathogenicity. Toxins of TA systems use diverse strategies to control bacterial growth and represent attractive therapeutic targets to fight pathogens. In this work, we have investigated the toxic mechanism of the three RelE toxins of Mycobacterium tuberculosis, the bacterium responsible for tuberculosis in humans. Structural studies showed that RelBE1, RelBE2, and RelBE3 TA complexes share conserved structural motifs distinct from the RelBE complex of Escherichia coli. Although RelE homologs have previously been reported to perform ribosome-dependent messenger RNA (mRNA) cleavage, detection of cleavage products by nEMOTE demonstrated that only RelE3 targets mRNA. In contrast, in vitro and in vivo analyses using Mycobacterium smegmatis and M. tuberculosis revealed that RelE1 is a site-specific RNase, able to cleave 16S rRNA from free 30S and formed 70S ribosomes, to release the anti-Shine-Dalgarno region and prevent translation. This stunning mode of action, which is likely shared with RelE2, demonstrates that there is broader diversity for toxic mechanisms within the widespread RelE family.
- Laboratoire de Microbiologie et Génétique Moléculaires (LMGM), Centre de Biologie Intégrative (CBI), Université de Toulouse, CNRS, 31062, Toulouse, France.
Organizational Affiliation: