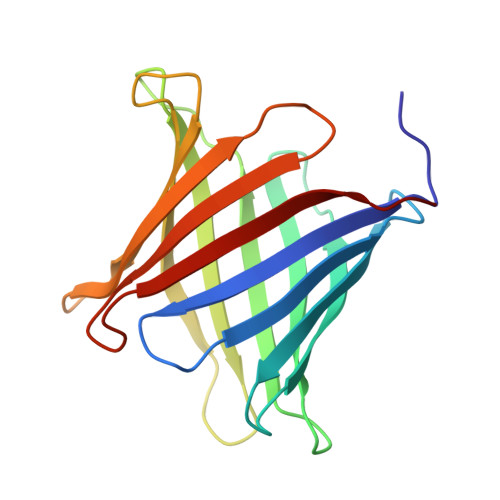
Sculpting conducting nanopore size and shape through de novo protein design.
Berhanu, S., Majumder, S., Muentener, T., Whitehouse, J., Berner, C., Bera, A.K., Kang, A., Liang, B., Khan, N., Sankaran, B., Tamm, L.K., Brockwell, D.J., Hiller, S., Radford, S.E., Baker, D., Vorobieva, A.A.(2024) Science 385: 282-288
- PubMed: 39024453
- DOI: https://doi.org/10.1126/science.adn3796
- Primary Citation of Related Structures:
8UZL, 9FDG - PubMed Abstract:
Transmembrane β-barrels have considerable potential for a broad range of sensing applications. Current engineering approaches for nanopore sensors are limited to naturally occurring channels, which provide suboptimal starting points. By contrast, de novo protein design can in principle create an unlimited number of new nanopores with any desired properties. Here we describe a general approach to designing transmembrane β-barrel pores with different diameters and pore geometries. Nuclear magnetic resonance and crystallographic characterization show that the designs are stably folded with structures resembling those of the design models. The designs have distinct conductances that correlate with their pore diameter, ranging from 110 picosiemens (~0.5 nanometer pore diameter) to 430 picosiemens (~1.1 nanometer pore diameter). Our approach opens the door to the custom design of transmembrane nanopores for sensing and sequencing applications.
- Department of Biochemistry, The University of Washington, Seattle, WA, USA.
Organizational Affiliation: