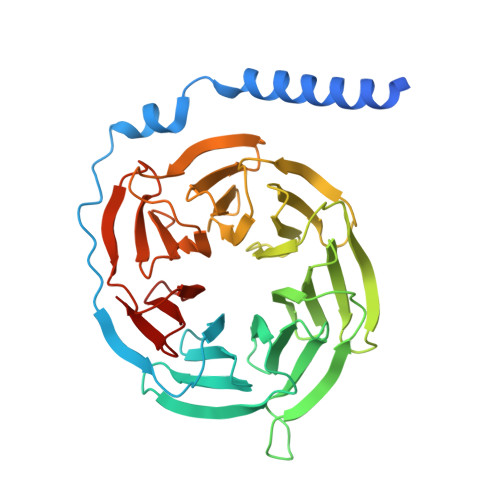
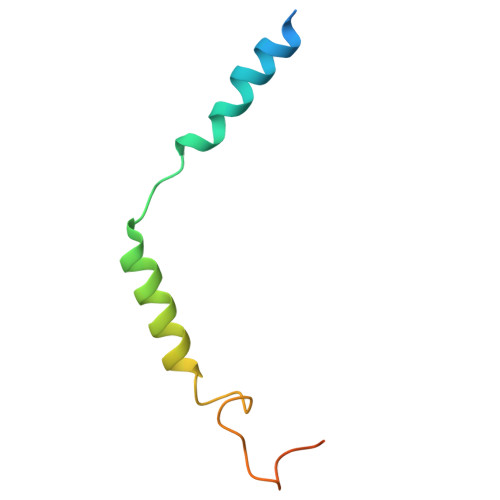
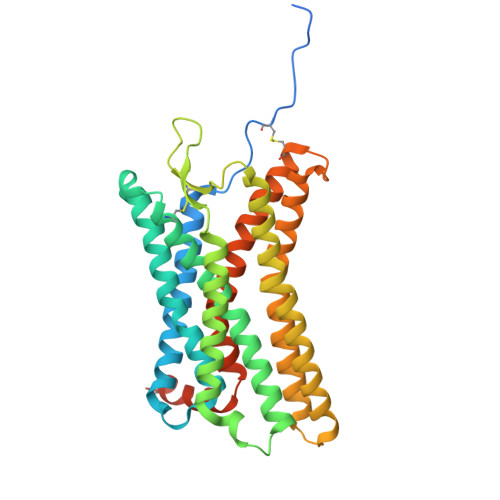
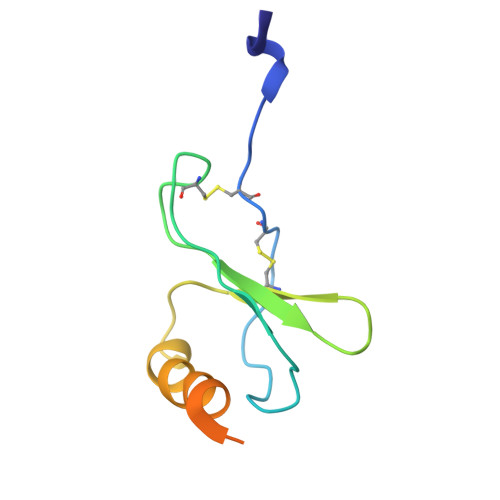
Structural basis for ligand promiscuity and high signaling activity of Kaposi's Sarcoma-associated Herpesvirus-encoded GPCR.
Park, J.B., Sahoo, B., Sahoo, A.R., Kim, D., Seo, H.D., Bowman, J., Kwak, M.J., Suh, S., Buck, M., Dai, X., Jung, J.U.(2025) Nat Commun 16: 8403-8403
- PubMed: 40998787
- DOI: https://doi.org/10.1038/s41467-025-63457-4
- Primary Citation of Related Structures:
8W1A, 9EJC - PubMed Abstract:
Kaposi's Sarcoma-associated Herpesvirus encodes ORF74, a viral G protein-coupled receptor homologous to CXCR2, which plays a crucial role in Kaposi's Sarcoma development through its high basal signaling activity. Our cryoEM analysis of ORF74 in ligand-free, BRIL-fused ligand-free, and CXCL1/Gi trimer -bound forms elucidates its ligand-independent signaling activity. A widely open, static extracellular cavity facilitates ligand promiscuity by enabling dynamic access and diverse binding modes. Structural alterations in CWxP, E/DRY, and NPxxY micro-switches stabilize the active conformation, leading to constitutive signaling. Metadynamics simulations reveal a dynamic ensemble between local switch structures corresponding to the inactive and active states, supporting spontaneous activation. CXCR2-ORF74 chimeras highlight intracellular loops 2 and 3 as key modulators of basal and agonist-induced activity. This study defines the structural basis of ORF74's ligand promiscuity, spontaneous activation, and high basal signaling, providing insights into its role in viral oncogenesis.
- Cancer Biology Department, Infection Biology Program, and Global Center for Pathogen and Human Health Research, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA.
Organizational Affiliation: