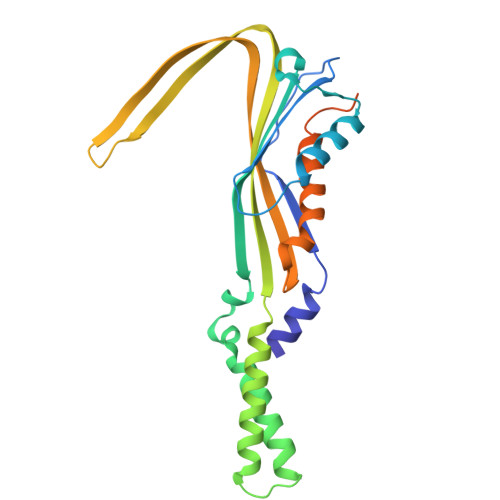
Structure of the conjugation surface exclusion protein TraT.
Chen, N., Bukys, A., Lundgren, C.A.K., Deme, J.C., El Sayyed, H., Kapanidis, A.N., Lea, S.M., Berks, B.C.(2025) Commun Biol 8: 1702-1702
- PubMed: 41299015
- DOI: https://doi.org/10.1038/s42003-025-09102-8
- Primary Citation of Related Structures:
9E2V - PubMed Abstract:
Conjugal transfer of plasmids between bacteria is a major route for the spread of antimicrobial resistance. Many conjugative plasmids encode exclusion systems that inhibit redundant conjugation. In incompatibility group F (IncF) plasmids surface exclusion is mediated by the outer membrane protein TraT. Here we report the cryoEM structure of the TraT exclusion protein complex from the canonical F plasmid of Escherichia coli. TraT is a hollow homodecamer shaped like a chef's hat. In contrast to most outer membrane proteins, TraT spans the outer membrane using transmembrane α-helices. We develop a microscopy-based conjugation assay to probe the effects of directed mutagenesis on TraT. Our analysis provides no support for the idea that TraT has specific interactions with partner proteins. Instead, we infer that TraT is most likely to function by physical interference with conjugation. This work provides structural insight into a natural inhibitor of microbial gene transfer.
- Department of Biochemistry, University of Oxford, Oxford, UK.
Organizational Affiliation: