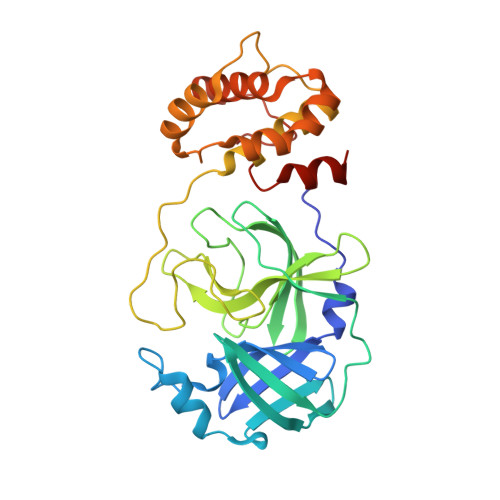
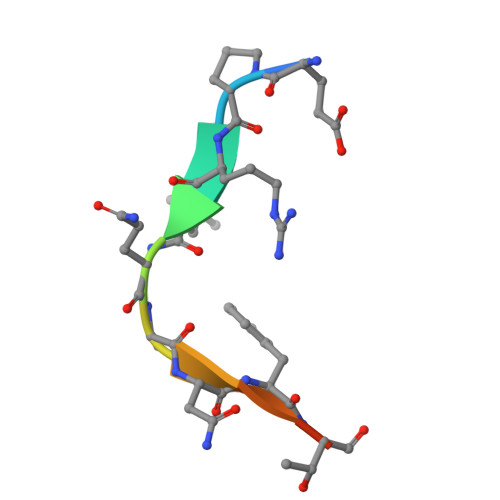
Recognition and cleavage of human tRNA methyltransferase TRMT1 by the SARS-CoV-2 main protease.
D'Oliviera, A., Dai, X., Mottaghinia, S., Olson, S., Geissler, E.P., Etienne, L., Zhang, Y., Mugridge, J.S.(2025) Elife 12
- PubMed: 39773525
- DOI: https://doi.org/10.7554/eLife.91168
- Primary Citation of Related Structures:
9DW6 - PubMed Abstract:
The SARS-CoV-2 main protease (M pro or Nsp5) is critical for production of viral proteins during infection and, like many viral proteases, also targets host proteins to subvert their cellular functions. Here, we show that the human tRNA methyltransferase TRMT1 is recognized and cleaved by SARS-CoV-2 M pro . TRMT1 installs the N 2 , N 2 -dimethylguanosine (m2,2G) modification on mammalian tRNAs, which promotes cellular protein synthesis and redox homeostasis. We find that M pro can cleave endogenous TRMT1 in human cell lysate, resulting in removal of the TRMT1 zinc finger domain. Evolutionary analysis shows the TRMT1 cleavage site is highly conserved in mammals, except in Muroidea, where TRMT1 is likely resistant to cleavage. TRMT1 proteolysis results in reduced tRNA binding and elimination of tRNA methyltransferase activity. We also determined the structure of an M pro -TRMT1 peptide complex that shows how TRMT1 engages the M pro active site in an uncommon substrate binding conformation. Finally, enzymology and molecular dynamics simulations indicate that kinetic discrimination occurs during a later step of M pro -mediated proteolysis following substrate binding. Together, these data provide new insights into substrate recognition by SARS-CoV-2 M pro that could help guide future antiviral therapeutic development and show how proteolysis of TRMT1 during SARS-CoV-2 infection impairs both TRMT1 tRNA binding and tRNA modification activity to disrupt host translation and potentially impact COVID-19 pathogenesis or phenotypes.
- Department of Chemistry & Biochemistry, University of Delaware, Newark, United States.
Organizational Affiliation: