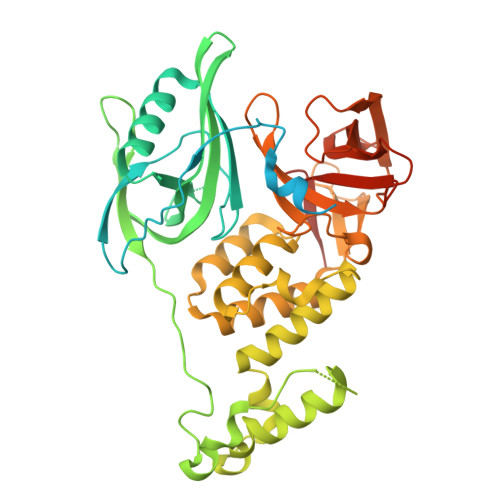
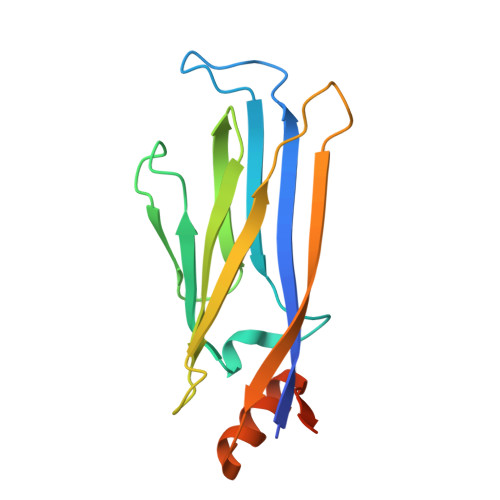
High-throughput diversification of protein-ligand surfaces to discover chemical inducers of proximity.
Shaum, J.B., Munoz I Ordono, M., Steen, E.A., Wenge, D.V., Cheong, H., Hunkeler, M., Bilotta, E.M., Rutter, Z., Barta, P.A., Thornhill, A.M., Milosevich, N., Hargis, L.M., Janowski, J., Bishop, T.R., Carter, T.R., da Camara, B., Hinterndorfer, M., Dada, L., He, W.J., Offensperger, F., Furihata, H., Schweber, S.R., Hatton, C., Wen, Y., Cravatt, B.F., Engle, K.M., Donovan, K.A., Melillo, B., Kitamura, S., Ciulli, A., Armstrong, S.A., Fischer, E.S., Winter, G.E., Erb, M.A.(2025) bioRxiv
- PubMed: 40950085
- DOI: https://doi.org/10.1101/2024.09.30.615685
- Primary Citation of Related Structures:
9DUR, 9GY3 - PubMed Abstract:
Chemical inducers of proximity (CIPs) stabilize biomolecular interactions, often causing an emergent rewiring of cellular biochemistry. While rational design strategies can expedite the discovery of heterobifunctional CIPs, monovalent, molecular glue-like CIPs have relied predominantly on serendipity. Envisioning a prospective approach to discover molecular glues for a pre-selected target, we hypothesized that pre-existing ligands could be systematically decorated with chemical modifications to empirically discover protein-ligand surfaces that are tuned to cooperatively engage another protein interface. Here, we used sulfur(VI)-fluoride exchange (SuFEx)-based high-throughput chemistry (HTC) to install 3,163 structurally diverse chemical building blocks onto ENL and BRD4 ligands and then screened the crude products for degrader activity. This revealed dHTC1, a potent, selective, and stereochemistry-dependent degrader of ENL. It recruits CRL4 CRBN to ENL through an extended interface of protein-protein and protein-ligand contacts, but only after pre-forming the ENL:dHTC1 complex. We also characterized two structurally distinct BRD4 degraders, including dHTC3, a molecular glue that selectively dimerizes the first bromodomain of BRD4 to SCF FBXO3 , an E3 ligase not previously accessible for chemical rewiring. Altogether, this study introduces HTC as a facile tool to discover new CIPs and actionable cellular effectors of proximity pharmacology.