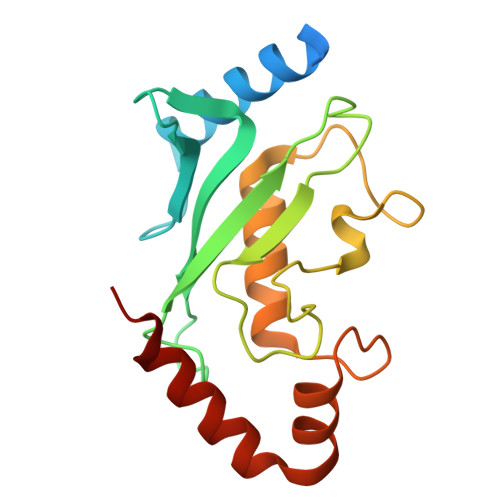
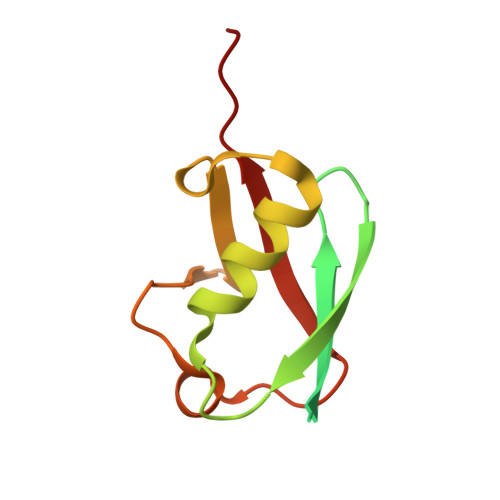
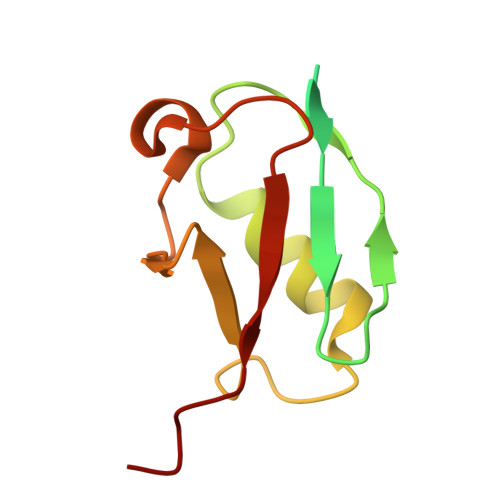
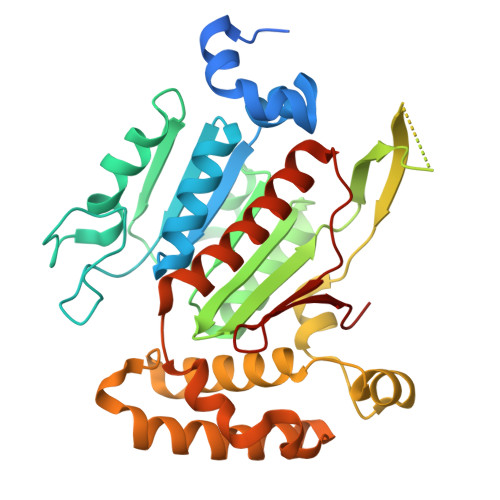
Cryo-EM structures reveal the molecular mechanism of SUMO E1-E2 thioester transfer.
Nayak, A., Nayak, D., Jia, L., Ruben, E.A., Viswanadhapalli, S., Dos Santos Bury, P., Nassar, K.M., Yu, C.H., Tumanova, A.A., Stratton, C.M., Ebadi, P., Ivanov, D.N., Sung, P., Vadlamudi, R.K., Wasmuth, E.V., Olsen, S.K.(2025) Nat Struct Mol Biol 32: 2441-2453
- PubMed: 40999065
- DOI: https://doi.org/10.1038/s41594-025-01681-8
- Primary Citation of Related Structures:
9DQB, 9DRJ - PubMed Abstract:
Post-translational modification of proteins by SUMO (small ubiquitin-like modifier) regulates fundamental cellular processes and occurs through the sequential interactions and activities of three enzymes: E1, E2 and E3. SUMO E1 activates SUMO in a two-step process involving adenylation and thioester bond formation, followed by transfer of SUMO to its dedicated E2 enzyme, UBC9. This process is termed E1-E2 thioester transfer (or transthioesterification). Despite its fundamental importance, the molecular basis for SUMO E1-UBC9 thioester transfer and the molecular rules governing SUMO E1-UBC9 specificity are poorly understood. Here we present cryo-EM reconstructions of human SUMO E1 in complex with UBC9, SUMO1 adenylate and SUMO1 thioester intermediate. Our structures reveal drastic conformational changes that accompany thioester transfer, providing insights into the molecular recognition of UBC9 by SUMO E1 and delineating the rules that govern SUMO E1-UBC9 specificity. Collectively, our structural, biochemical and cell-based studies elucidate the molecular mechanisms by which SUMOylation exerts its essential biological functions.
- Department of Biochemistry and Structural Biology and Greehey Children's Cancer Research Institute, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA.
Organizational Affiliation: