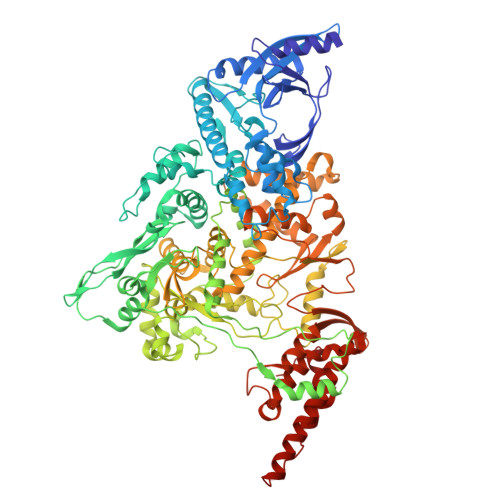
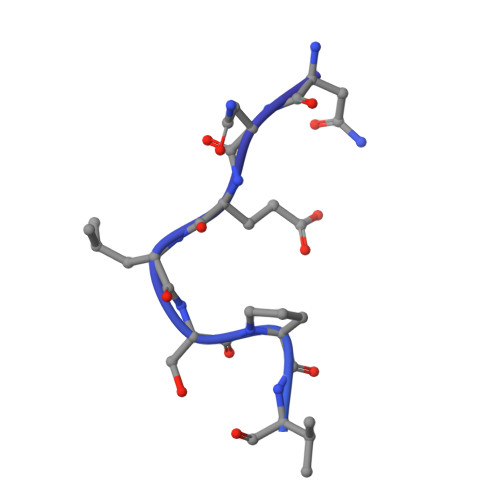
The mechanism for GTP-mediated RNA capping by the SARS-CoV-2 NiRAN domain remains unresolved.
Small, G.I., Darst, S.A., Campbell, E.A.(2025) Cell 188: 4456-4461.e6
- PubMed: 40570834
- DOI: https://doi.org/10.1016/j.cell.2025.05.044
- Primary Citation of Related Structures:
9DJ8 - PubMed Abstract:
The Nidovirus RdRp-associated nucleotidyltransferase (NiRAN) domain initiates mRNA capping in coronaviruses through a GDP-polyribonucleotidyltransferase reaction, with RNA covalently linked to nsp9. GDP is the preferred substrate for this reaction, but the NiRAN domain can also utilize GTP to produce an authentic 5' RNA cap structure, though the GTP-mediated mechanism is unclear. Yan and colleagues claimed to have delineated the reaction mechanism from the analysis of a cryoelectron microscopy (cryo-EM) structure of a trapped catalytic intermediate of the SARS-CoV-2 NiRAN domain with a β-γ-non-hydrolyzable GTP analog (GMPPNP) and RNA-nsp9 (PDB: 8GWE). We show that the cryo-EM data used to derive PDB: 8GWE do not support the presence of GMPPNP in the NiRAN active site, and the resulting atomic model is incompatible with fundamental chemical principles. We conclude that Yan and colleagues' conclusions are not experimentally supported and the mechanism for GTP-mediated RNA capping by the SARS-CoV-2 NiRAN domain remains unresolved. This Matters Arising paper is in response to Yan et al. (2022), published in Cell. See also the response by Huang et al. (2025), published in this issue.
- Laboratory of Molecular Pathogenesis, the Rockefeller University, 1230 York Avenue, New York, NY 10065, USA; Laboratory of Molecular Biophysics, the Rockefeller University, 1230 York Avenue, New York, NY 10065, USA.
Organizational Affiliation: