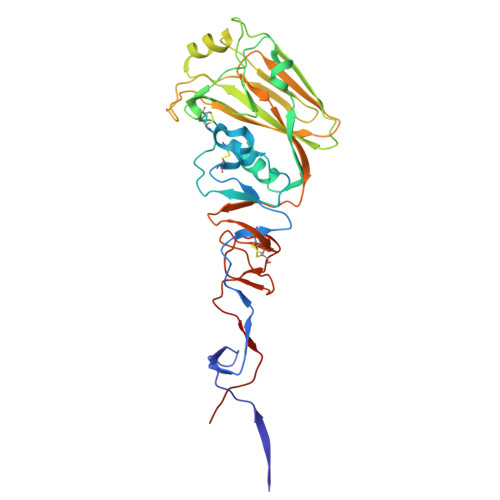
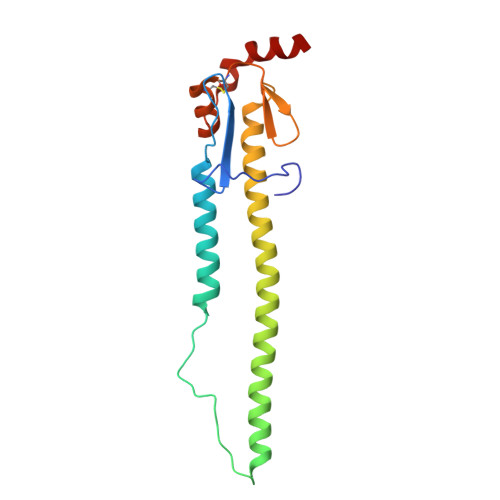
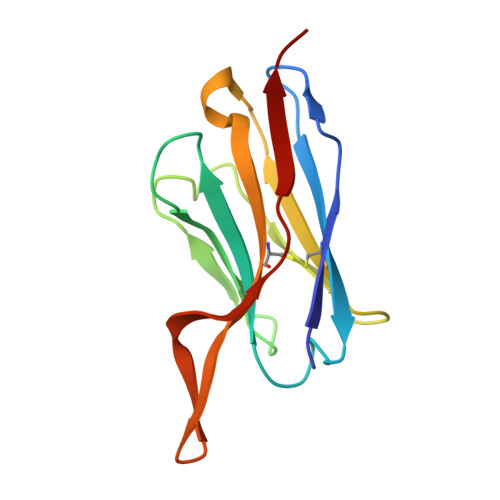
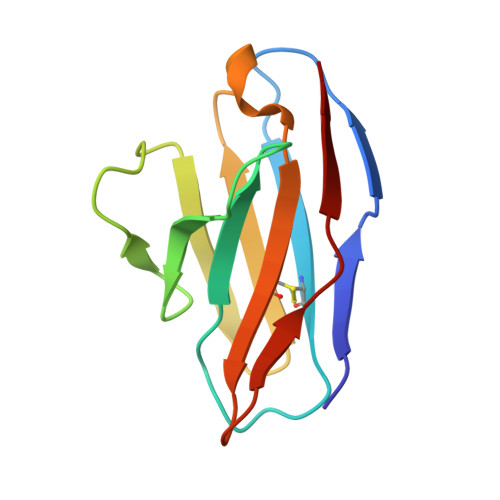
High-throughput synthesis and specificity characterization of natively paired influenza hemagglutinin antibodies with oPool + display.
Ouyang, W.O., Lv, H., Liu, W., Lei, R., Mou, Z., Pholcharee, T., Talmage, L., Tong, M., Ji, W., Wang, Y., Dailey, K.E., Gopal, A.B., Choi, D., Ardagh, M.R., Rodriguez, L.A., Guthmiller, J.J., Dai, X., Wu, N.C.(2025) Sci Transl Med 17: eadt4147-eadt4147
- PubMed: 40901925
- DOI: https://doi.org/10.1126/scitranslmed.adt4147
- Primary Citation of Related Structures:
9CU7, 9DBX - PubMed Abstract:
Antibody discovery is crucial for developing therapeutics and vaccines and for understanding adaptive immunity. However, the lack of approaches to synthesize antibodies with defined sequences in a high-throughput manner represents a major bottleneck in antibody discovery. Here, we present oPool + display, a high-throughput cell-free platform that combined oligo pool synthesis and mRNA display to rapidly construct and characterize hundreds to thousands of natively paired antibodies in parallel. As a proof of concept, we applied oPool + display to probe the binding specificity of more than 300 uncommon influenza hemagglutinin-specific antibodies against nine hemagglutinin variants through 16 screens. More than 5000 binding tests were performed in 3 to 5 days of hands-on time with further scaling potential. Follow-up structural and functional analysis of two antibodies revealed the versatility of the human immunoglobulin gene segment D3-3 ( IGHD-3-3 ) in recognizing the hemagglutinin stem. Overall, this study established an experimental platform that not only accelerates antibody characterization but also enables unbiased discovery of recurring molecular signatures among antibodies with the same specificity.
- Department of Biochemistry, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
Organizational Affiliation: