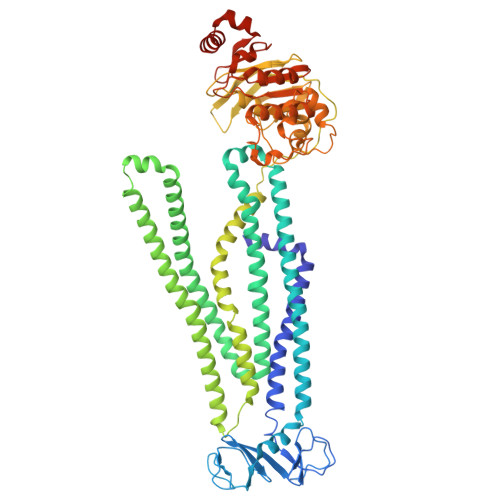
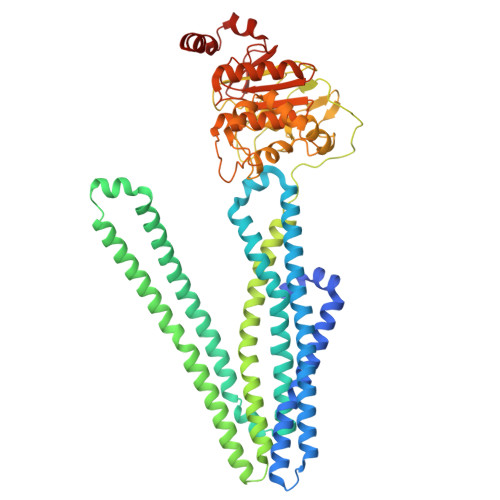
Drug-bound outward-facing conformation of a heterodimeric ABC exporter suggests a putative mechanism of drug translocation.
Tang, Q., Sinclair, M., Bisignano, P., Zhang, Y., Tajkhorshid, E., Mchaourab, H.S.(2025) Nat Commun 16: 10403-10403
- PubMed: 41285748
- DOI: https://doi.org/10.1038/s41467-025-65318-6
- Primary Citation of Related Structures:
9CUP, 9CUR, 9CUS - PubMed Abstract:
Multidrug transport by ATP binding cassette (ABC) exporters entails a mechanism to modulate drug affinity across the transport cycle. Here, we combine cryo-EM and molecular dynamics (MD) simulations to illuminate a lipid-competition mechanism to drive substrate translocation by ABC exporters. We determine cryo-EM structures of the ABC transporter BmrCD in drug-loaded inward-facing (IF) and outward-facing (OF) conformations in lipid nanodiscs to reveal the structural basis of alternating access, details of drug-transporter interactions, and the scale of drug movement between the two conformations. Remarkably, the structures uncover lipid molecules bound in or near the transporter vestibule along with the drugs. MD trajectories from the IF structure show that these lipids stimulate drug disorder and translocation towards the innermost constricted region of the vestibule. Similarly, bound lipids enter the OF vestibule and weaken drug-transporter interactions facilitating drug release. Our results complete a near-atomic model of BmrCD's conformational cycle and suggest the modulation of substrate-transporter interactions by lipids.
- Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, TN, USA. qingyu.tang@vanderbilt.edu.
Organizational Affiliation: