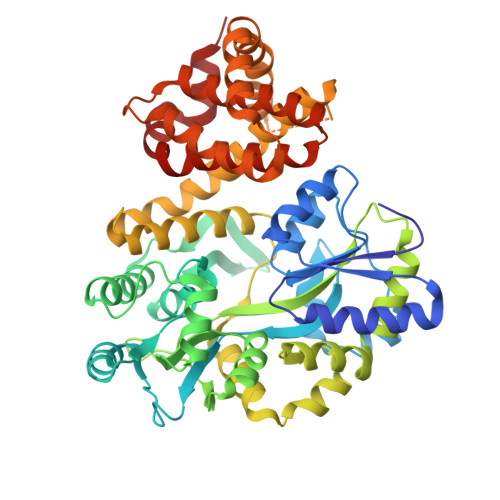
Structural basis of BAK sequestration by MCL-1 in apoptosis.
Srivastava, S., Sekar, G., Ojoawo, A., Aggarwal, A., Ferreira, E., Uchikawa, E., Yang, M., Grace, C.R., Dey, R., Lin, Y.L., Guibao, C.D., Jayaraman, S., Mukherjee, S., Kossiakoff, A.A., Dong, B., Myasnikov, A., Moldoveanu, T.(2025) Mol Cell 85: 1606-1623.e10
- PubMed: 40187349
- DOI: https://doi.org/10.1016/j.molcel.2025.03.013
- Primary Citation of Related Structures:
9CPE, 9CPF, 9CPH, 9CPN - PubMed Abstract:
Apoptosis controls cell fate, ensuring tissue homeostasis and promoting disease when dysregulated. The rate-limiting step in apoptosis is mitochondrial poration by the effector B cell lymphoma 2 (BCL-2) family proteins BAK and BAX, which are activated by initiator BCL-2 homology 3 (BH3)-only proteins (e.g., BIM) and inhibited by guardian BCL-2 family proteins (e.g., MCL-1). We integrated structural, biochemical, and pharmacological approaches to characterize the human prosurvival MCL-1:BAK complex assembled from their BCL-2 globular core domains. We reveal a canonical interaction with BAK BH3 bound to the hydrophobic groove of MCL-1 and disordered and highly dynamic BAK regions outside the complex interface. We predict similar conformations of activated effectors in complex with other guardians or effectors. The MCL-1:BAK complex is a major cancer drug target. We show that MCL-1 inhibitors are inefficient in neutralizing the MCL-1:BAK complex, requiring high doses to initiate apoptosis. Our study underscores the need to design superior clinical candidate MCL-1 inhibitors.
- Biochemistry and Molecular Biology, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Organizational Affiliation: