In silico Self-assembling Protein Nanoparticles Optimization for Protein Antigen display
Cappelli, L., Wahome, N., Cinelli, P., Giusti, F., Seraj, N., Cartocci, E., Harshbarger, W., Delany, I., Cozzi, R.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Bacterial non-heme ferritin | 163 | Escherichia coli | Mutation(s): 0 Gene Names: ftnA, Z2960, ECs2613 EC: 1.16.3.2 | 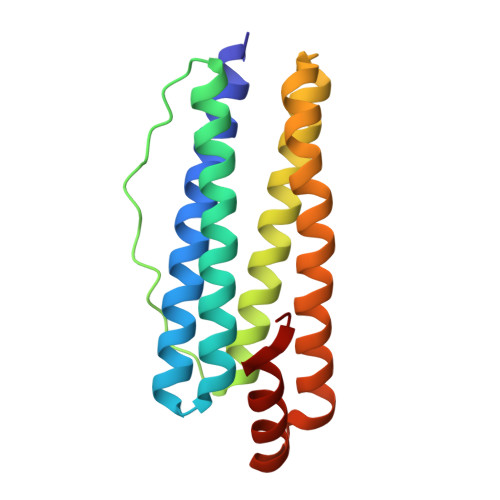 | |
UniProt | |||||
Find proteins for P0A998 (Escherichia coli (strain K12)) Explore P0A998 Go to UniProtKB: P0A998 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0A998 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 180.373 | α = 90 |
| b = 180.373 | β = 90 |
| c = 180.373 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data scaling |
| PHASER | phasing |
| Coot | model building |
| HKL-2000 | data reduction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Other private | United States | -- |