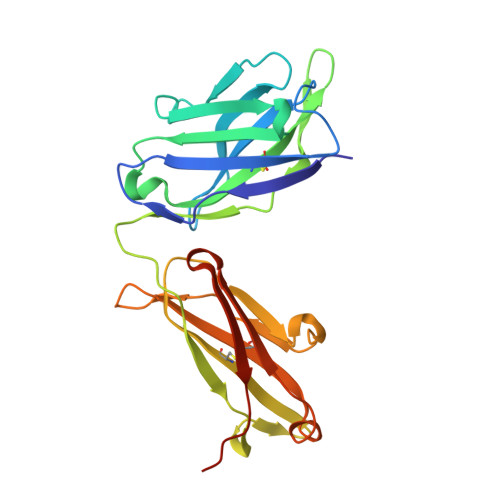
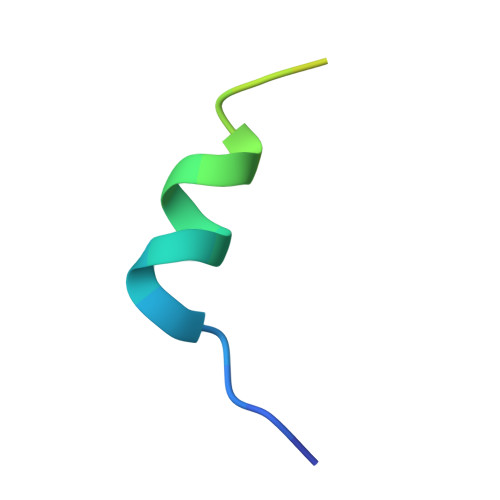
Isolation and structure of broad SIV-neutralizing antibodies reveal a proximal helical MPER epitope recognized by a rhesus multi-donor class.
Gorman, J., Du, R., Lai, Y.T., Ahmadi, M.S., King, H.A.D., Song, K., Manalang, K., Gonelli, C.A., Schramm, C.A., Cheng, C., Nguyen, R., Ambrozak, D., Druz, A., Shen, C.H., Yang, Y., Douek, D.C., Kwong, P.D., Roederer, M., Mason, R.D.(2025) Cell Rep 44: 115163-115163
- PubMed: 39792559
- DOI: https://doi.org/10.1016/j.celrep.2024.115163
- Primary Citation of Related Structures:
9BLX, 9BNS, 9BP1 - PubMed Abstract:
The membrane-proximal external region (MPER) of the HIV-1 envelope is a target for broadly neutralizing antibodies (bnAbs), and vaccine-elicited MPER-directed antibodies have recently been reported from a human clinical trial. In this study, we sought to identify MPER-directed nAbs in simian immunodeficiency virus (SIV)-infected rhesus macaques. We isolated four lineages of SIV MPER-directed nAbs from two SIV-infected macaques. The nAbs displayed low potency but up to 90% breadth on a 20-strain SIV panel. Crystal structures of representative nAbs in complex with SIV MPER peptides revealed the SIV antibodies to bind a helical epitope at the N-terminal (proximal) region of the MPER, defining a reproducible multi-donor class encompassing all four lineages. HIV-1 comparison showed that this class of SIV MPER-directed antibodies targets a helical region overlapping that targeted by human vaccine-elicited ones. Thus, a prevalent and reproducible class of SIV bnAbs recognizes an epitope similar to that recently observed in an HIV-1-vaccine trial.
- Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892, USA; Division of Viral Products, Center for Biologics Evaluation and Research, Food and Drug Administration, Silver Spring, MD 20993, USA. Electronic address: jason.gorman@fda.hhs.gov.
Organizational Affiliation: