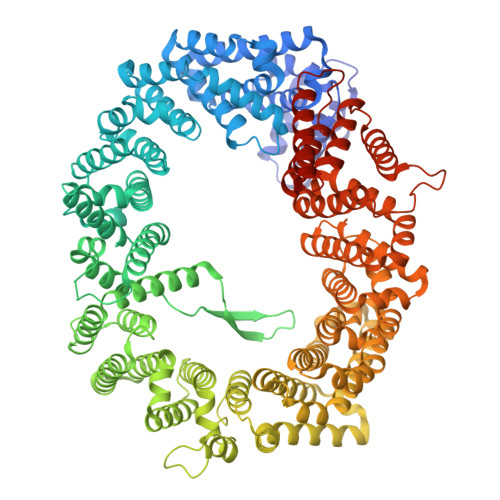
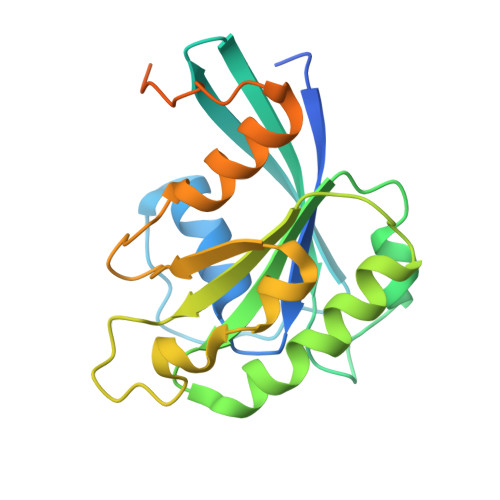
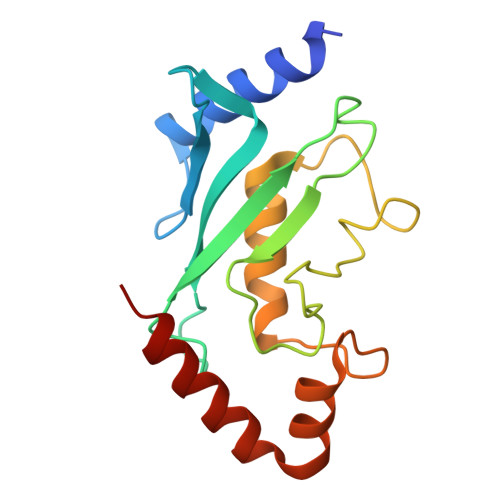
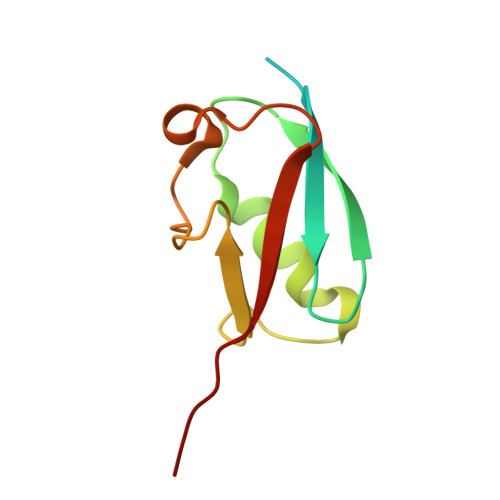
Structural basis for a nucleoporin exportin complex between RanBP2, SUMO1-RanGAP1, the E2 Ubc9, Crm1 and the Ran GTPase.
Baytshtok, V., DiMattia, M.A., Lima, C.D.(2025) Nat Commun 16: 6403-6403
- PubMed: 40640138
- DOI: https://doi.org/10.1038/s41467-025-61694-1
- Primary Citation of Related Structures:
9B62 - PubMed Abstract:
The human nucleoporin RanBP2/Nup358 interacts with SUMO1-modified RanGAP1 and the SUMO E2 Ubc9 at the nuclear pore complex (NPC) to promote export and disassembly of exportin Crm1/Ran(GTP)/cargo complexes. In mitosis, RanBP2/SUMO1-RanGAP1/Ubc9 remains intact after NPC disassembly and is recruited to kinetochores and mitotic spindles by Crm1 where it contributes to mitotic progression. RanBP2 binds SUMO1-RanGAP1/Ubc9 via motifs that also catalyze SUMO E3 ligase activity. Here, we resolve cryo-EM structures of a RanBP2 C-terminal fragment in complex with Crm1, SUMO1-RanGAP1/Ubc9, and two molecules of Ran(GTP). These structures reveal several interactions with Crm1 including a nuclear export signal (NES) for RanGAP1, the deletion of which mislocalizes RanGAP1 and the Ran GTPase in cells. Our structural and biochemical results support models in which RanBP2 E3 ligase activity is dependent on Crm1, the RanGAP1 NES and Ran GTPase cycling.
- Structural Biology Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY, USA.
Organizational Affiliation: