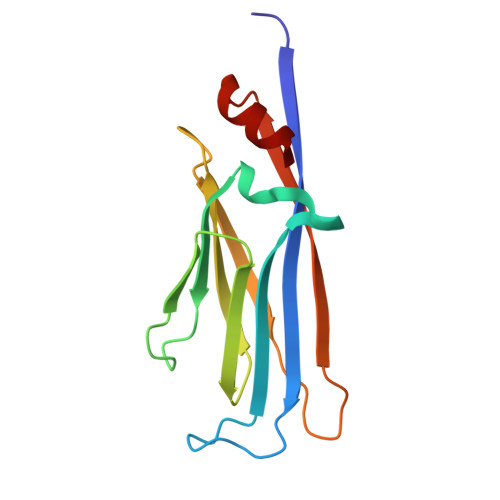
A multivalent engagement of ENL with MOZ.
Becht, D.C., Selvam, K., Lachance, C., Cote, V., Li, K., Nguyen, M.C., Pareek, A., Shi, X., Wen, H., Blanco, M.A., Cote, J., Kutateladze, T.G.(2025) Nat Struct Mol Biol 32: 709-718
- PubMed: 39794553
- DOI: https://doi.org/10.1038/s41594-024-01455-8
- Primary Citation of Related Structures:
9ARO, 9ARR - PubMed Abstract:
The epigenetic cofactor ENL (eleven-nineteen-leukemia) and the acetyltransferase MOZ (monocytic leukemia zinc finger) have vital roles in transcriptional regulation and are implicated in aggressive forms of leukemia. Here, we describe the mechanistic basis for the intertwined association of ENL and MOZ. Genomic analysis shows that ENL and MOZ co-occupy active promoters and that MOZ recruits ENL to its gene targets. Structural studies reveal a multivalent assembly of ENL at the intrinsically disordered region (IDR) of MOZ. While the extraterminal (ET) domain of ENL recognizes the canonical ET-binding motif in IDR, the YEATS domains of ENL and homologous AF9 bind to a set of acetylation sites in the MOZ IDR that are generated by the acetyltransferase CBP (CREB-binding protein). Our findings suggest a multifaceted acetylation-dependent and independent coupling of ENL, MOZ and CBP/p300, which may contribute to leukemogenic activities of the ENL-MOZ assembly and chromosomal translocations of ENL, MOZ and CBP/p300.
- Department of Pharmacology, University of Colorado School of Medicine, Aurora, CO, USA.
Organizational Affiliation: