Insights About the Structure and Recognition Mechanism of the Rev Response Element of HIV-1 Revealed by FRET-monitored Mutagenesis and Modeling
Szewczyk, M.P., Loharch, S., Lopez-Nunez, S., Gallego J.(2025) J Mol Biol 437
- PubMed: 40976347
- DOI: https://doi.org/10.1016/j.jmb.2025.169451
- Primary Citation of Related Structures:
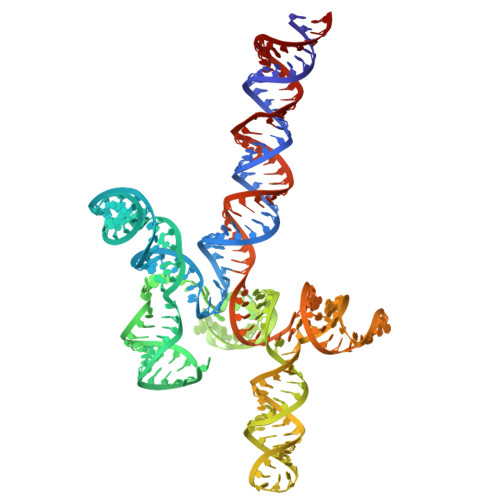
9A9V - PubMed Abstract:
The complex between several monomers of the HIV-1 Rev protein and the Rev Response Element (RRE) in the viral RNA allows nuclear export of unspliced or singly-spliced viral transcripts, an essential step in the virus cycle that is not targeted by any of the currently marketed antiretroviral treatments. The RRE adopts a multi-domain structure whose three-dimensional details are currently unknown at atomic resolution. In order to shed light on RRE structure and on the mechanism of RRE-Rev complex assembly, we set up a method based on fluorescence resonance energy transfer (FRET) that has allowed us to measure RRE interdomain distance as a function of solution conditions and changes in helix length and junction nucleotides. By combining this information with all-atom ensemble modeling, comparative sequence analyses and electrophoretic band-shift assays assessing Rev association, we clarify the roles of helices and junctions on the spatial organization of RRE domains and RRE-Rev complex formation and propose an all-atom model of RRE structure consistent with the available evidence.
- Centro de Investigación Traslacional San Alberto Magno, Universidad Católica de Valencia 46001 Valencia, Spain; Escuela de Doctorado, Universidad Católica de Valencia 46001 Valencia, Spain.
Organizational Affiliation: