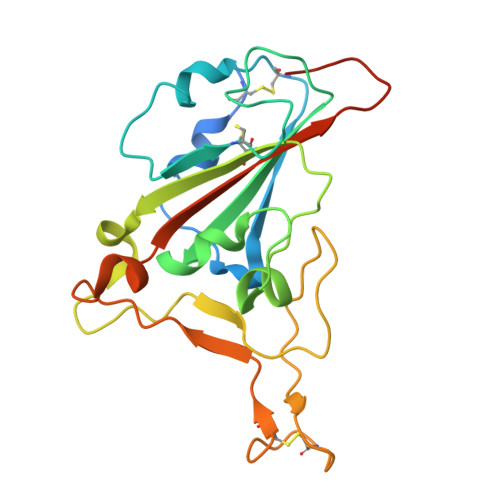
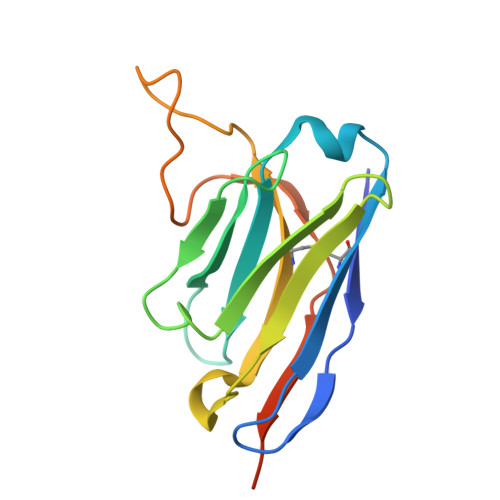
Identification of a potent SARS-CoV-2 neutralizing nanobody targeting the receptor-binding domain of the spike protein.
Liu, C., Hadiatullah, H., Yuchi, Z.(2024) Int J Biol Macromol 281: 136403-136403
- PubMed: 39383917
- DOI: https://doi.org/10.1016/j.ijbiomac.2024.136403
- Primary Citation of Related Structures:
8ZRD - PubMed Abstract:
SARS-CoV-2 and its variants continue to pose a significant threat to public health. Nanobodies (Nbs) that inhibit the interaction between the receptor-binding domain (RBD) of the spike protein and the host cell receptor angiotensin-converting enzyme 2 (ACE2) are promising drug candidates. In this study, we report the discovery and structural characterization of a potent Nb that targets the RBD. By screening a phage display alpaca naive Nbs library using the RBD as bait, we identified sixteen candidate Nbs. Of these, nine exhibited nanomolar to micromolar binding affinity and strong neutralizing activity against pseudotyped SARS-CoV-2 viruses, with NbS4 showing the highest neutralization potency. The crystal structure of the SARS-CoV-2 RBD in complex with NbS4 revealed that this Nb binds to a site partially overlapping the ACE2 binding region. Importantly, the key binding residues of NbS4 in the RBD are conserved across most known variants, making it a promising candidate for COVID-19 treatment.
- Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, Frontiers Science Center for Synthetic Biology, School of Pharmaceutical Science and Technology, Faculty of Medicine, Tianjin University, Tianjin 300072, China; Haihe Laboratory of Sustainable Chemical Transformations, Tianjin 300192, China.
Organizational Affiliation: