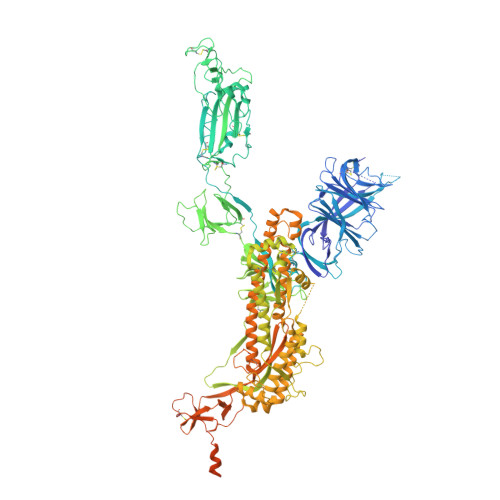
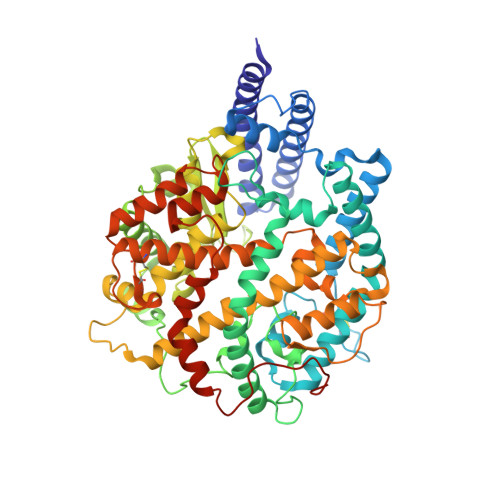
Altered receptor binding, antibody evasion and retention of T cell recognition by the SARS-CoV-2 XBB.1.5 spike protein.
Mannar, D., Saville, J.W., Poloni, C., Zhu, X., Bezeruk, A., Tidey, K., Ahmed, S., Tuttle, K.S., Vahdatihassani, F., Cholak, S., Cook, L., Steiner, T.S., Subramaniam, S.(2024) Nat Commun 15: 1854-1854
- PubMed: 38424106
- DOI: https://doi.org/10.1038/s41467-024-46104-2
- Primary Citation of Related Structures:
8VKK, 8VKL, 8VKM, 8VKN, 8VKO, 8VKP - PubMed Abstract:
The XBB.1.5 variant of SARS-CoV-2 has rapidly achieved global dominance and exhibits a high growth advantage over previous variants. Preliminary reports suggest that the success of XBB.1.5 stems from mutations within its spike glycoprotein, causing immune evasion and enhanced receptor binding. We present receptor binding studies that demonstrate retention of binding contacts with the human ACE2 receptor and a striking decrease in binding to mouse ACE2 due to the revertant R493Q mutation. Despite extensive evasion of antibody binding, we highlight a region on the XBB.1.5 spike protein receptor binding domain (RBD) that is recognized by serum antibodies from a donor with hybrid immunity, collected prior to the emergence of the XBB.1.5 variant. T cell assays reveal high frequencies of XBB.1.5 spike-specific CD4 + and CD8 + T cells amongst donors with hybrid immunity, with the CD4 + T cells skewed towards a Th1 cell phenotype and having attenuated effector cytokine secretion as compared to ancestral spike protein-specific cells. Thus, while the XBB.1.5 variant has retained efficient human receptor binding and gained antigenic alterations, it remains susceptible to recognition by T cells induced via vaccination and previous infection.
- Department of Biochemistry and Molecular Biology, University of British Columbia, Vancouver, BC, V6T 1Z3, Canada.
Organizational Affiliation: