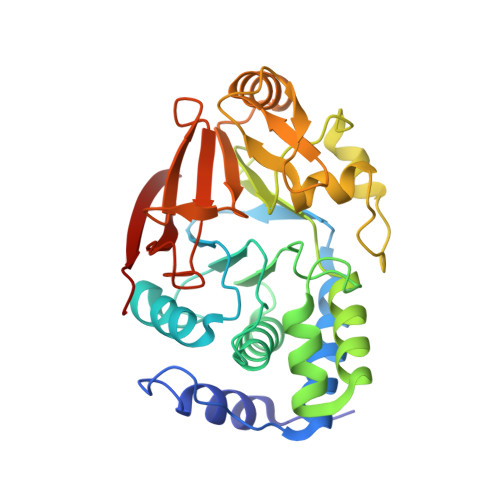
The SDS22:PP1:I3 complex: SDS22 binding to PP1 loosens the active site metal to prime metal exchange.
Choy, M.S., Srivastava, G., Robinson, L.C., Tatchell, K., Page, R., Peti, W.(2023) J Biological Chem 300: 105515-105515
- PubMed: 38042495
- DOI: https://doi.org/10.1016/j.jbc.2023.105515
- Primary Citation of Related Structures:
8U5G - PubMed Abstract:
SDS22 and Inhibitor-3 (I3) are two ancient regulators of protein phosphatase 1 (PP1) that regulate multiple essential biological processes. Both SDS22 and I3 form stable dimeric complexes with PP1; however, and atypically for PP1 regulators, they also form a triple complex, where both proteins bind to PP1 simultaneously (SPI complex). Here we report the crystal structure of the SPI complex. While both regulators bind PP1 in conformations identical to those observed in their individual PP1 complexes, PP1 adopts the SDS22-bound conformation, which lacks its M1 metal. Unexpectedly, surface plasmon resonance (SPR) revealed that the affinity of I3 for the SDS22:PP1 complex is ∼10-fold lower than PP1 alone. We show that this change in binding affinity is solely due to the interaction of I3 with the PP1 active site, specifically PP1's M2 metal, demonstrating that SDS22 likely allows for PP1 M2 metal exchange and thus PP1 biogenesis.
- Department of Molecular Biology and Biophysics, University of Connecticut Health Center, Farmington, Connecticut, USA.
Organizational Affiliation: