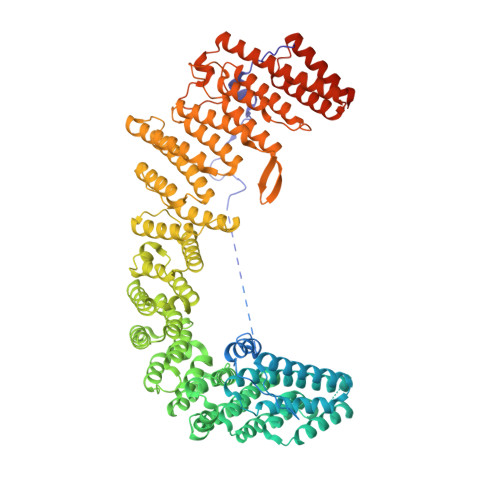
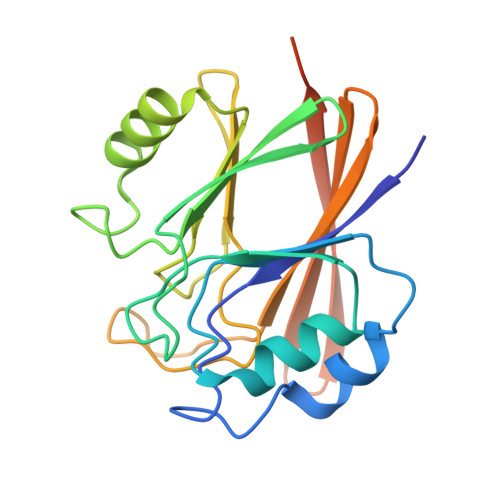
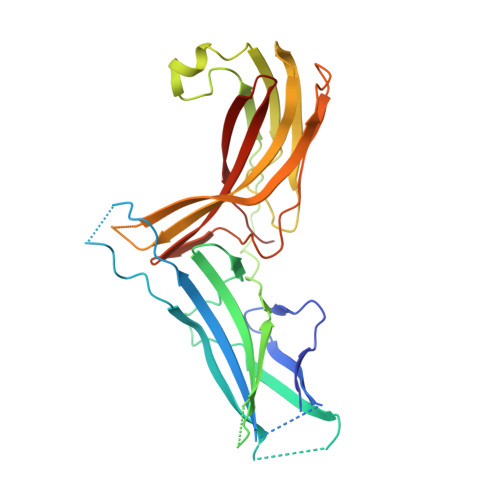
Structural organization of the retriever-CCC endosomal recycling complex.
Boesch, D.J., Singla, A., Han, Y., Kramer, D.A., Liu, Q., Suzuki, K., Juneja, P., Zhao, X., Long, X., Medlyn, M.J., Billadeau, D.D., Chen, Z., Chen, B., Burstein, E.(2024) Nat Struct Mol Biol 31: 910-924
- PubMed: 38062209
- DOI: https://doi.org/10.1038/s41594-023-01184-4
- Primary Citation of Related Structures:
8SYM, 8SYN, 8SYO - PubMed Abstract:
The recycling of membrane proteins from endosomes to the cell surface is vital for cell signaling and survival. Retriever, a trimeric complex of vacuolar protein-sorting-associated protein (VPS)35L, VPS26C and VPS29, together with the CCC complex comprising coiled-coil domain-containing (CCDC)22, CCDC93 and copper metabolism domain-containing (COMMD) proteins, plays a crucial role in this process. The precise mechanisms underlying retriever assembly and its interaction with CCC have remained elusive. Here, we present a high-resolution structure of retriever in humans determined using cryogenic electron microscopy. The structure reveals a unique assembly mechanism, distinguishing it from its remotely related paralog retromer. By combining AlphaFold predictions and biochemical, cellular and proteomic analyses, we further elucidate the structural organization of the entire retriever-CCC complex across evolution and uncover how cancer-associated mutations in humans disrupt complex formation and impair membrane protein homeostasis. These findings provide a fundamental framework for understanding the biological and pathological implications associated with retriever-CCC-mediated endosomal recycling.
- Roy J. Carver Department of Biochemistry, Biophysics and Molecular Biology, Iowa State University, Ames, IA, USA.
Organizational Affiliation: