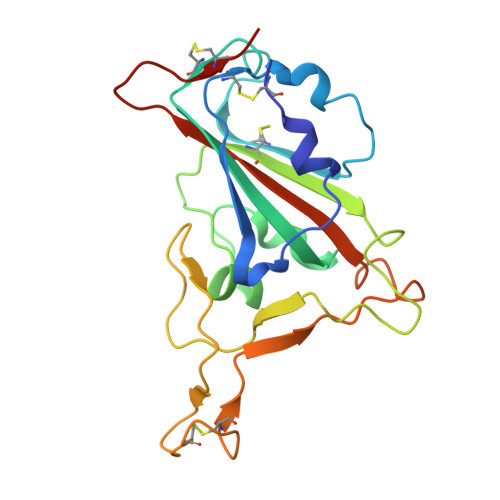
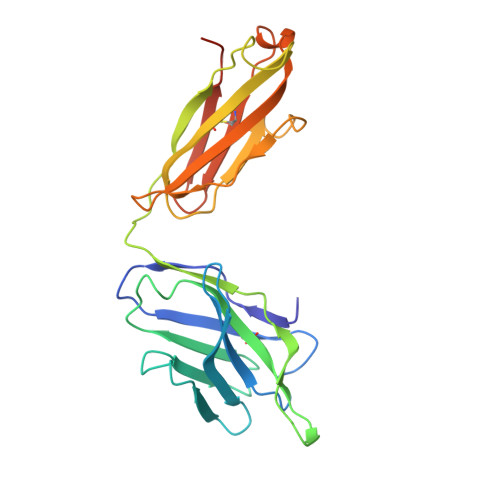
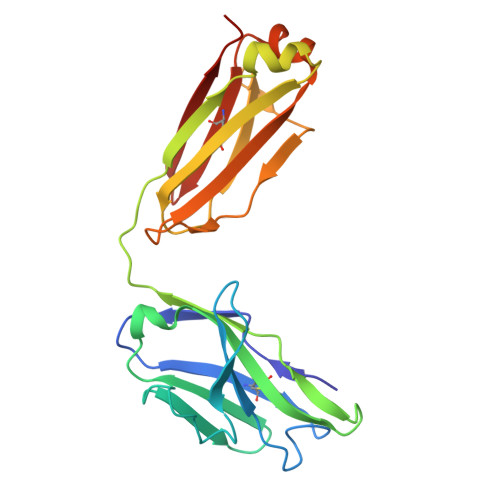
A human monoclonal antibody neutralizing SARS-CoV-2 Omicron variants containing the L452R mutation.
Stein, S.C., Hansen, G., Ssebyatika, G., Stroh, L.J., Ochulor, O., Herold, E., Schwarzloh, B., Mutschall, D., Zischke, J., Cordes, A.K., Schneider, T., Hinrichs, I., Blasczyk, R., Kleine-Weber, H., Hoffmann, M., Klein, F., Kaiser, F.K., Gonzalez-Hernandez, M., Armando, F., Ciurkiewicz, M., Beythien, G., Pohlmann, S., Baumgartner, W., Osterhaus, A., Schulz, T.F., Krey, T.(2024) J Virol 98: e0122324-e0122324
- PubMed: 39494911
- DOI: https://doi.org/10.1128/jvi.01223-24
- Primary Citation of Related Structures:
8RRN - PubMed Abstract:
The effectiveness of SARS-CoV-2 therapeutic antibodies targeting the spike (S) receptor-binding domain (RBD) has been hampered by the emergence of variants of concern (VOCs), which have acquired mutations to escape neutralizing antibodies (nAbs). These mutations are not evenly distributed on the RBD surface but cluster on several distinct surfaces, suggesting an influence of the targeted epitope on the capacity to neutralize a broad range of VOCs. Here, we identified a potent nAb from convalescent patients targeting the receptor-binding domain of a broad range of SARS-CoV-2 VOCs. Except for the Lambda and BA.2.86 variants, this nAb efficiently inhibited the entry of most tested VOCs, including Omicron subvariants BA.1, BA.2, XBB.1.5, and EG.5.1 and to a limited extent also BA.4/5, BA.4.6, and BQ.1.1. It bound recombinant S protein with picomolar affinity, reduced the viral load in the lung of infected hamsters, and prevented the severe lung pathology typical for SARS-CoV-2 infections. An X-ray structure of the nAb-RBD complex revealed an epitope that does not fall into any of the conventional classes and provided insights into its broad neutralization properties. Our findings highlight a conserved epitope within the SARS-CoV-2 RBD that should be preferably targeted by therapeutic antibodies and inform rational vaccine development.IMPORTANCETherapeutic antibodies are effective in preventing severe disease from SARS-CoV-2 infection and constitute an important option in pandemic preparedness, but mutations within the S protein of virus variants (e.g., a mutation of L452) confer resistance to many of such antibodies. Here, we identify a human antibody targeting the S protein receptor-binding domain (RBD) with an elevated escape barrier and characterize its interaction with the RBD functionally and structurally at the atomic level. A direct comparison with reported antibodies targeting the same epitope illustrates important differences in the interface, providing insights into the breadth of antibody binding. These findings highlight the relevance of an extended neutralization profiling in combination with biochemical and structural characterization of the antibody-RBD interaction for the selection of future therapeutic antibodies, which may accelerate the control of potential future pandemics.
- Institute of Virology, Hannover Medical School, Hannover, Germany.
Organizational Affiliation: