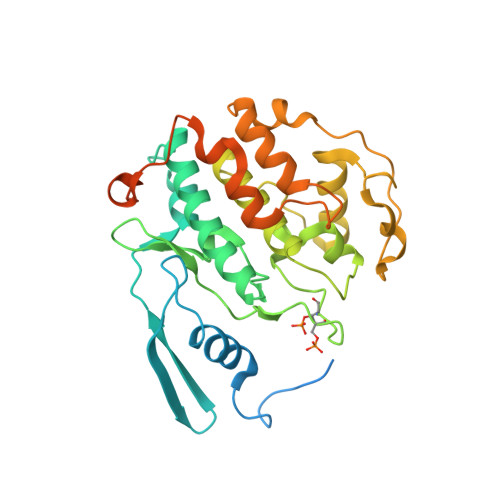
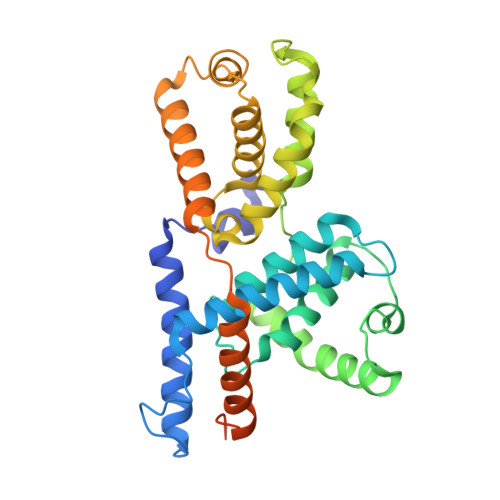
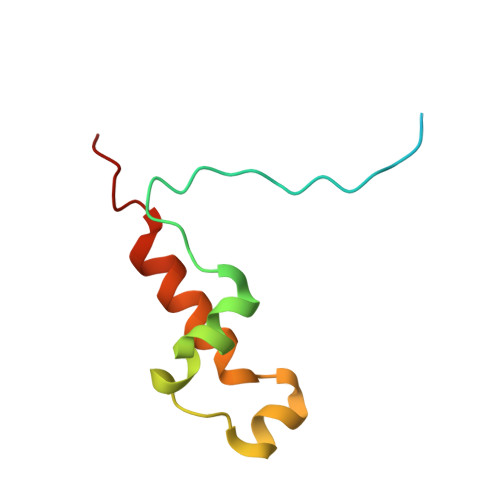
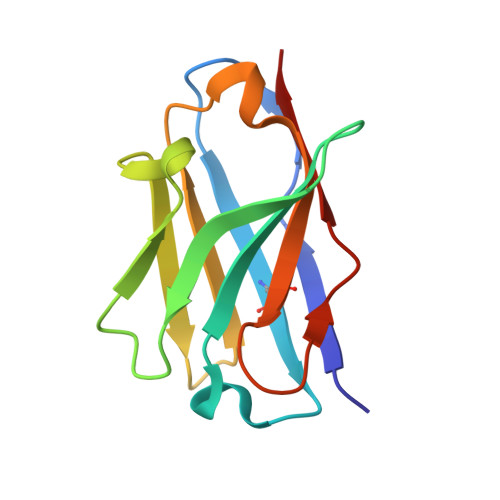
Structural basis of Cdk7 activation by dual T-loop phosphorylation.
Duster, R., Anand, K., Binder, S.C., Schmitz, M., Gatterdam, K., Fisher, R.P., Geyer, M.(2024) Nat Commun 15: 6597-6597
- PubMed: 39097586
- DOI: https://doi.org/10.1038/s41467-024-50891-z
- Primary Citation of Related Structures:
8PYR - PubMed Abstract:
Cyclin-dependent kinase 7 (Cdk7) is required in cell-cycle and transcriptional regulation owing to its function as both a CDK-activating kinase (CAK) and part of transcription factor TFIIH. Cdk7 forms active complexes by associating with Cyclin H and Mat1, and is regulated by two phosphorylations in the activation segment (T loop): the canonical activating modification at T170 and another at S164. Here we report the crystal structure of the human Cdk7/Cyclin H/Mat1 complex containing both T-loop phosphorylations. Whereas pT170 coordinates basic residues conserved in other CDKs, pS164 nucleates an arginine network unique to the ternary Cdk7 complex, involving all three subunits. We identify differential dependencies of kinase activity and substrate recognition on the individual phosphorylations. CAK function is unaffected by T-loop phosphorylation, whereas activity towards non-CDK substrates is increased several-fold by T170 phosphorylation. Moreover, dual T-loop phosphorylation stimulates multisite phosphorylation of the RNA polymerase II (RNAPII) carboxy-terminal domain (CTD) and SPT5 carboxy-terminal repeat (CTR) region. In human cells, Cdk7 activation is a two-step process wherein S164 phosphorylation precedes, and may prime, T170 phosphorylation. Thus, dual T-loop phosphorylation can regulate Cdk7 through multiple mechanisms, with pS164 supporting tripartite complex formation and possibly influencing processivity, while pT170 enhances activity towards key transcriptional substrates.
- Institute of Structural Biology, University of Bonn, Venusberg-Campus 1, 53127, Bonn, Germany.
Organizational Affiliation: