Anaerobic fixed-target serial crystallography using sandwiched silicon nitride membranes.
Bjelcic, M., Sigfridsson Clauss, K.G.V., Aurelius, O., Milas, M., Nan, J., Ursby, T.(2023) Acta Crystallogr D Struct Biol 79: 1018-1025
- PubMed: 37860963
- DOI: https://doi.org/10.1107/S205979832300880X
- Primary Citation of Related Structures:
8PUQ, 8PUR - PubMed Abstract:
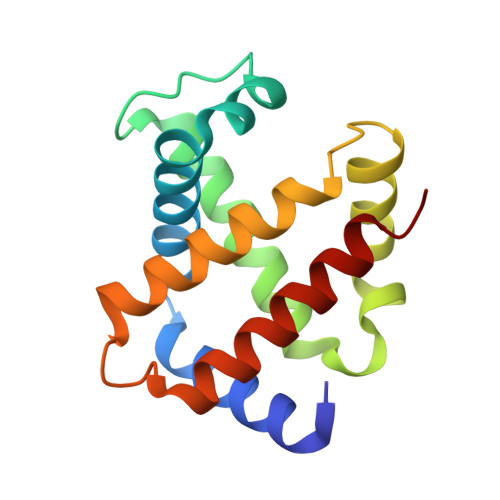
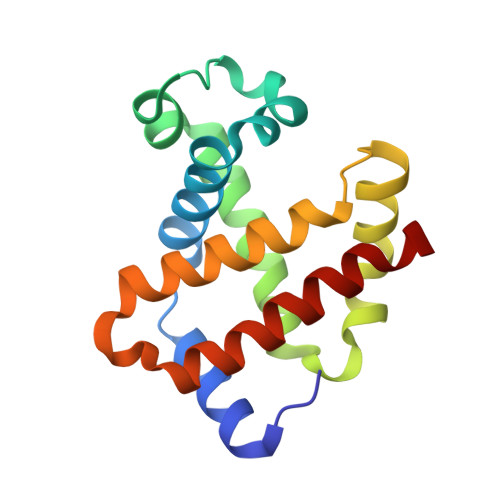
In recent years, the emergence of serial crystallography, initially pioneered at X-ray free-electron lasers (XFELs), has sparked a growing interest in collecting macromolecular crystallographic data at room temperature. Various fixed-target serial crystallography techniques have been developed, ranging from commercially available chips to in-house designs implemented at different synchrotron facilities. Nevertheless, there is currently no commercially available chip (known to the authors) specifically designed for the direct handling of oxygen-sensitive samples. This study presents a methodology employing silicon nitride chips arranged in a `sandwich' configuration, enabling reliable room-temperature data collection from oxygen-sensitive samples. The method involves the utilization of a custom-made 3D-printed assembling tool and a MX sample holder. To validate the effectiveness of the proposed method, deoxyhemoglobin and methemoglobin samples were investigated using the BioMAX X-ray macromolecular crystallography beamline, the Balder X-ray absorption spectroscopy beamline and UV-Vis absorption spectroscopy.
- MAX IV Laboratory, Lund University, PO Box 118, SE-221 00 Lund, Sweden.
Organizational Affiliation: