Structure-Guided Design of a Highly Potent Partial RXR Agonist with Superior Physicochemical Properties.
Lewandowski, M., Carmina, M., Knumann, L., Sai, M., Willems, S., Kasch, T., Pollinger, J., Knapp, S., Marschner, J.A., Chaikuad, A., Merk, D.(2024) J Med Chem 67: 2152-2164
- PubMed: 38237049
- DOI: https://doi.org/10.1021/acs.jmedchem.3c02095
- Primary Citation of Related Structures:
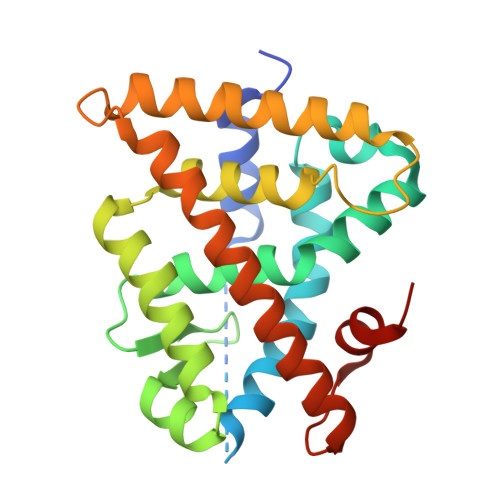
8PP0 - PubMed Abstract:
Retinoid X receptors (RXRs, NR2B1-3) hold therapeutic potential in oncology, neurodegeneration, and metabolic diseases, but traditional RXR agonists mimicking the natural ligand 9- cis retinoic acid exhibit poor physicochemical properties, pharmacokinetics, and safety profiles. Improved RXR ligands are needed to exploit RXR modulation as a promising therapeutic concept in various indications beyond its current role in second-line cancer treatment. Here, we report the co-crystal structure of RXR in complex with a novel pyrimidine-based ligand and the structure-informed optimization of this scaffold to highly potent and highly soluble RXR agonists. Focused structure-activity relationship elucidation and rigidization resulted in a substantially optimized partial RXR agonist with low nanomolar potency, no cytotoxic activity, and very favorable physicochemical properties highlighting this promising scaffold for the development of next-generation RXR targeting drugs.
- Department of Pharmacy, Ludwig-Maximilians-Universität (LMU) München, 81377 Munich, Germany.
Organizational Affiliation: