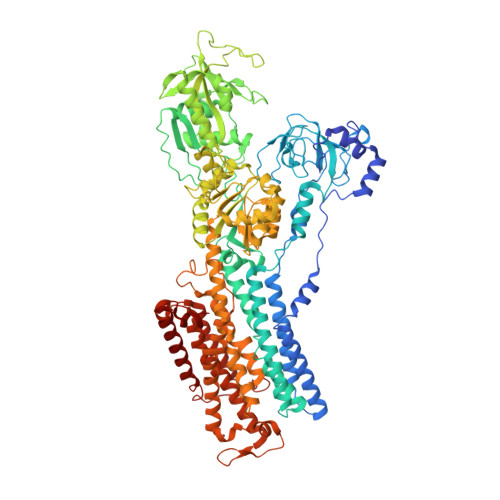
A Na pump with reduced stoichiometry is up-regulated by brine shrimp in extreme salinities.
Artigas, P., Meyer, D.J., Young, V.C., Spontarelli, K., Eastman, J., Strandquist, E., Rui, H., Roux, B., Birk, M.A., Nakanishi, H., Abe, K., Gatto, C.(2023) Proc Natl Acad Sci U S A 120: e2313999120-e2313999120
- PubMed: 38079564
- DOI: https://doi.org/10.1073/pnas.2313999120
- Primary Citation of Related Structures:
8K1L - PubMed Abstract:
Brine shrimp ( Artemia ) are the only animals to thrive at sodium concentrations above 4 M. Salt excretion is powered by the Na + ,K + -ATPase (NKA), a heterodimeric (αβ) pump that usually exports 3Na + in exchange for 2 K + per hydrolyzed ATP. Artemia express several NKA catalytic α-subunit subtypes. High-salinity adaptation increases abundance of α2 KK , an isoform that contains two lysines (Lys308 and Lys758 in transmembrane segments TM4 and TM5, respectively) at positions where canonical NKAs have asparagines ( Xenopus α1's Asn333 and Asn785). Using de novo transcriptome assembly and qPCR, we found that Artemia express two salinity-independent canonical α subunits (α1 NN and α3 NN ), as well as two β variants, in addition to the salinity-controlled α2 KK . These β subunits permitted heterologous expression of the α2 KK pump and determination of its CryoEM structure in a closed, ion-free conformation, showing Lys758 residing within the ion-binding cavity. We used electrophysiology to characterize the function of α2 KK pumps and compared it to that of Xenopus α1 (and its α2 KK -mimicking single- and double-lysine substitutions). The double substitution N333K/N785K confers α2 KK -like characteristics to Xenopus α1, and mutant cycle analysis reveals energetic coupling between these two residues, illustrating how α2 KK 's Lys308 helps to maintain high affinity for external K + when Lys758 occupies an ion-binding site. By measuring uptake under voltage clamp of the K + -congener 86 Rb + , we prove that double-lysine-substituted pumps transport 2Na + and 1 K + per catalytic cycle. Our results show how the two lysines contribute to generate a pump with reduced stoichiometry allowing Artemia to maintain steeper Na + gradients in hypersaline environments.
- Department of Cell Physiology and Molecular Biophysics, Center for Membrane Protein Research, Texas Tech University Health Sciences Center, Lubbock, TX 79430.
Organizational Affiliation: