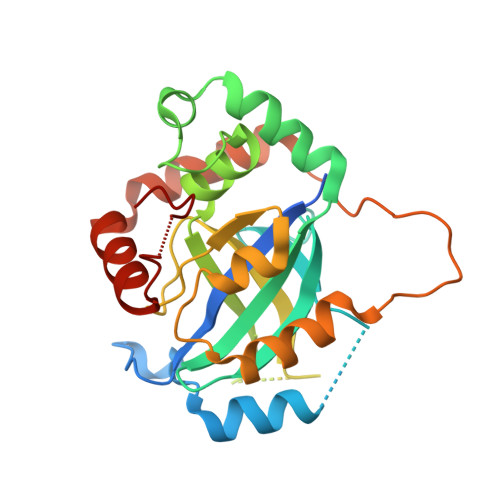
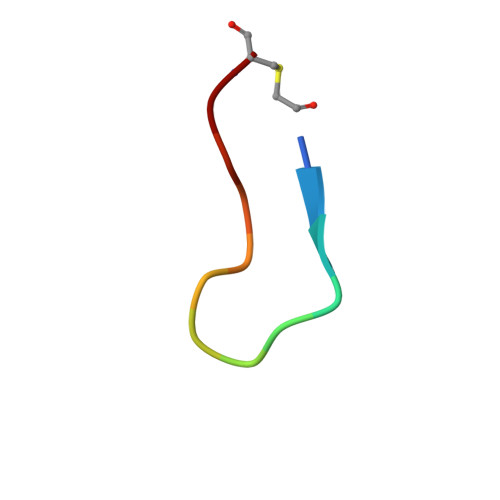
Peptide-to-Small Molecule: Discovery of Non-Covalent, Active-Site Inhibitors of beta-Herpesvirus Proteases.
Yoshida, S., Sako, Y., Nikaido, E., Ueda, T., Kozono, I., Ichihashi, Y., Nakahashi, A., Onishi, M., Yamatsu, Y., Kato, T., Nishikawa, J., Tachibana, Y.(2023) ACS Med Chem Lett 14: 1558-1566
- PubMed: 37974946
- DOI: https://doi.org/10.1021/acsmedchemlett.3c00359
- Primary Citation of Related Structures:
8J3S, 8J3T - PubMed Abstract:
Viral proteases, the key enzymes that regulate viral replication and assembly, are promising targets for antiviral drug discovery. Herpesvirus proteases are enzymes with no crystallographically confirmed noncovalent active-site binders, owing to their shallow and polar substrate-binding pockets. Here, we applied our previously reported "Peptide-to-Small Molecule" strategy to generate novel inhibitors of β-herpesvirus proteases. Rapid selection with a display technology was used to identify macrocyclic peptide 1 bound to the active site of human cytomegalovirus protease (HCMV Pro ) with high affinity, and pharmacophore queries were defined based on the results of subsequent intermolecular interaction analyses. Membrane-permeable small molecule 19 , designed de novo according to this hypothesis, exhibited enzyme inhibitory activity (IC 50 = 10 -6 to 10 -7 M) against β-herpesvirus proteases, and the design concept was proved by X-ray cocrystal analysis.
- Pharmaceutical Research Division, Shionogi Pharmaceutical Research Center, 3-1-1 Futaba-cho, Toyonaka, Osaka 561-0825, Japan.
Organizational Affiliation: