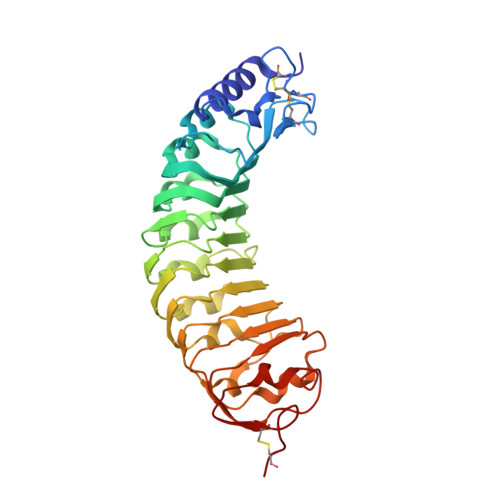
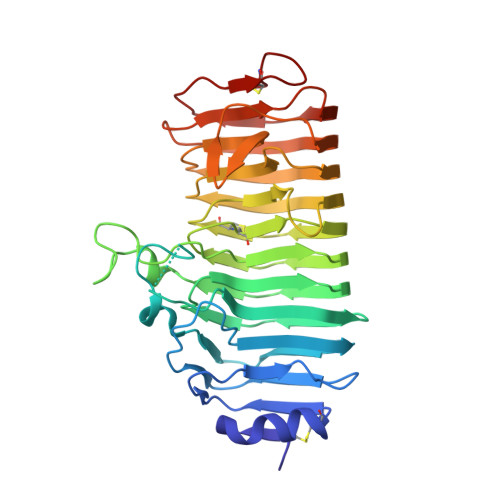
A plant mechanism of hijacking pathogen virulence factors to trigger innate immunity.
Xiao, Y., Sun, G., Yu, Q., Gao, T., Zhu, Q., Wang, R., Huang, S., Han, Z., Cervone, F., Yin, H., Qi, T., Wang, Y., Chai, J.(2024) Science 383: 732-739
- PubMed: 38359129
- DOI: https://doi.org/10.1126/science.adj9529
- Primary Citation of Related Structures:
8IKW, 8IKX - PubMed Abstract:
Polygalacturonase-inhibiting proteins (PGIPs) interact with pathogen-derived polygalacturonases to inhibit their virulence-associated plant cell wall-degrading activity but stimulate immunity-inducing oligogalacturonide production. Here we show that interaction between Phaseolus vulgaris PGIP2 (PvPGIP2) and Fusarium phyllophilum polygalacturonase (FpPG) enhances substrate binding, resulting in inhibition of the enzyme activity of FpPG. This interaction promotes FpPG-catalyzed production of long-chain immunoactive oligogalacturonides, while diminishing immunosuppressive short oligogalacturonides. PvPGIP2 binding creates a substrate binding site on PvPGIP2-FpPG, forming a new polygalacturonase with boosted substrate binding activity and altered substrate preference. Structure-based engineering converts a putative PGIP that initially lacks FpPG-binding activity into an effective FpPG-interacting protein. These findings unveil a mechanism for plants to transform pathogen virulence activity into a defense trigger and provide proof of principle for engineering PGIPs with broader specificity.
- Center for Plant Biology, School of Life Sciences, Tsinghua University, Beijing 100084, China.
Organizational Affiliation: