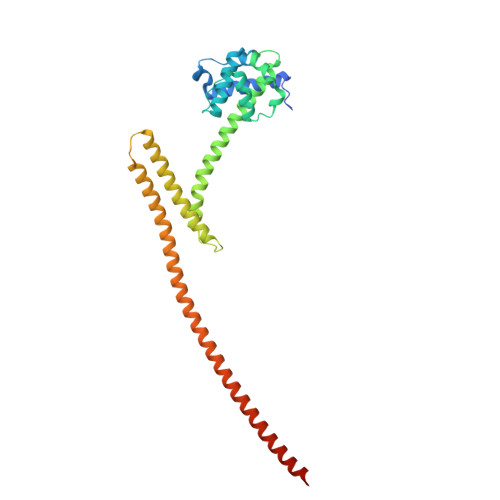
Structure of the Ndc80 complex and its interactions at the yeast kinetochore-microtubule interface.
Zahm, J.A., Jenni, S., Harrison, S.C.(2023) Open Biol 13: 220378-220378
- PubMed: 36883282
- DOI: https://doi.org/10.1098/rsob.220378
- Primary Citation of Related Structures:
8G0P, 8G0Q - PubMed Abstract:
The conserved Ndc80 kinetochore complex, Ndc80c, is the principal link between mitotic spindle microtubules and centromere-associated proteins. We used AlphaFold 2 (AF2) to obtain predictions of the Ndc80 'loop' structure and of the Ndc80 : Nuf2 globular head domains that interact with the Dam1 subunit of the heterodecameric DASH/Dam1 complex (Dam1c). The predictions guided design of crystallizable constructs, with structures close to the predicted ones. The Ndc80 'loop' is a stiff, α-helical 'switchback' structure; AF2 predictions and positions of preferential cleavage sites indicate that flexibility within the long Ndc80c rod occurs instead at a hinge closer to the globular head. Conserved stretches of the Dam1 C terminus bind Ndc80c such that phosphorylation of Dam1 serine residues 257, 265 and 292 by the mitotic kinase Ipl1/Aurora B can release this contact during error correction of mis-attached kinetochores. We integrate the structural results presented here into our current molecular model of the kinetochore-microtubule interface. The model illustrates how multiple interactions between Ndc80c, DASH/Dam1c and the microtubule lattice stabilize kinetochore attachments.
- Department of Biological Chemistry and Molecular Pharmacology, and.
Organizational Affiliation: