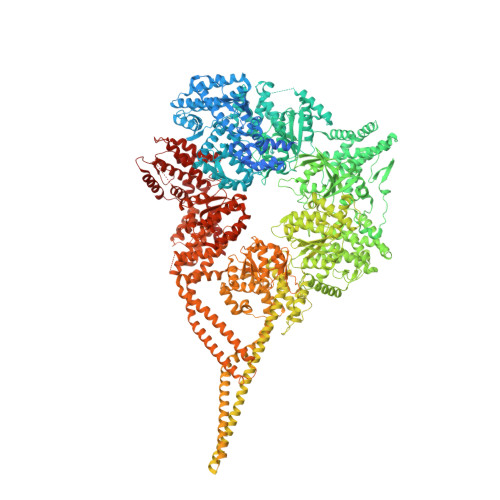
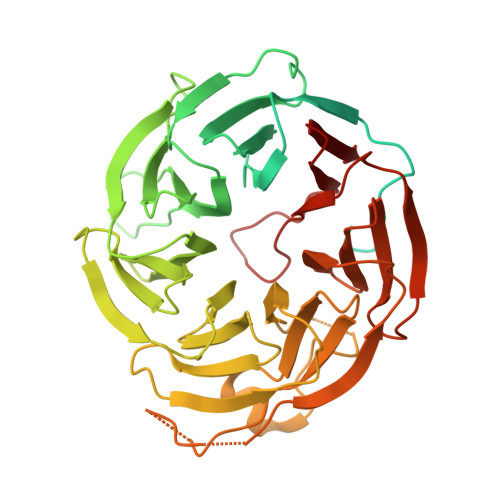
Lis1 relieves cytoplasmic dynein-1 autoinhibition by acting as a molecular wedge.
Karasmanis, E.P., Reimer, J.M., Kendrick, A.A., Nguyen, K.H.V., Rodriguez, J.A., Truong, J.B., Lahiri, I., Reck-Peterson, S.L., Leschziner, A.E.(2023) Nat Struct Mol Biol 30: 1357-1364
- PubMed: 37620585
- DOI: https://doi.org/10.1038/s41594-023-01069-6
- Primary Citation of Related Structures:
8DZZ, 8E00 - PubMed Abstract:
Cytoplasmic dynein-1 transports intracellular cargo towards microtubule minus ends. Dynein is autoinhibited and undergoes conformational changes to form an active complex that consists of one or two dynein dimers, the dynactin complex, and activating adapter(s). The Lissencephaly 1 gene, LIS1, is genetically linked to the dynein pathway from fungi to mammals and is mutated in people with the neurodevelopmental disease lissencephaly. Lis1 is required for active dynein complexes to form, but how it enables this is unclear. Here, we present a structure of two yeast dynein motor domains with two Lis1 dimers wedged in-between. The contact sites between dynein and Lis1 in this structure, termed 'Chi,' are required for Lis1's regulation of dynein in Saccharomyces cerevisiae in vivo and the formation of active human dynein-dynactin-activating adapter complexes in vitro. We propose that this structure represents an intermediate in dynein's activation pathway, revealing how Lis1 relieves dynein's autoinhibited state.
- Department of Cellular and Molecular Medicine, University of California San Diego, La Jolla, CA, USA.
Organizational Affiliation: