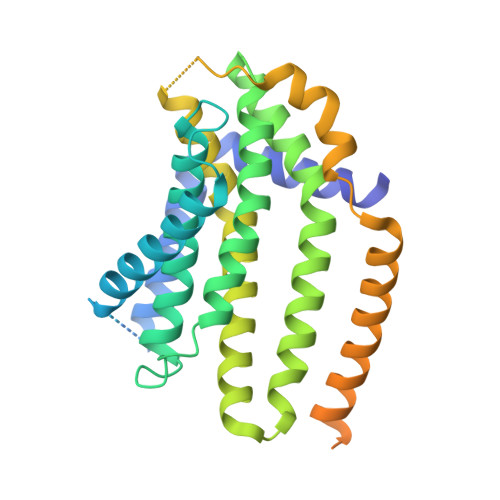
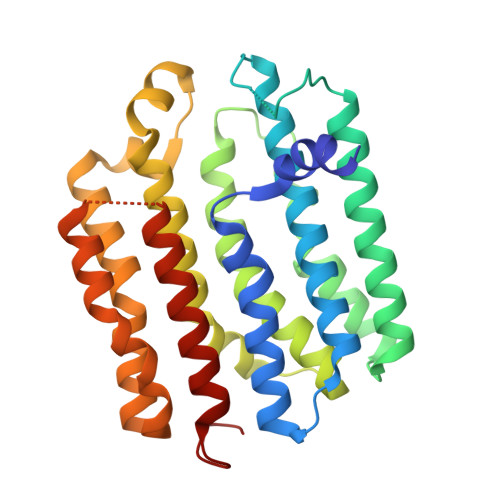
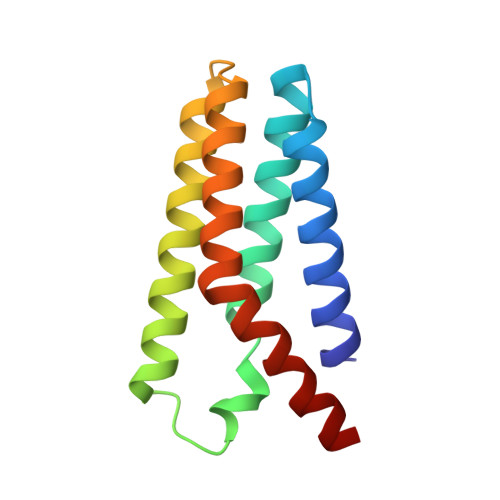
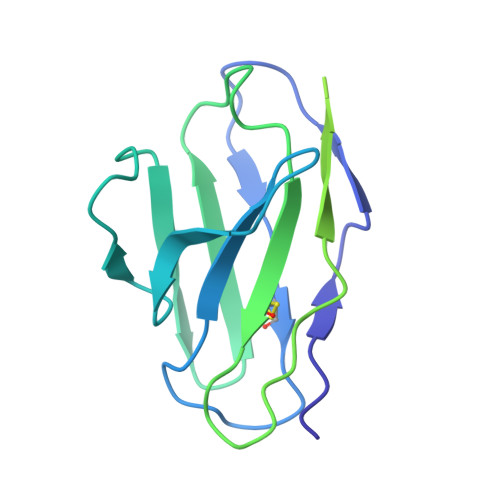
Regulated degradation of HMG CoA reductase requires conformational changes in sterol-sensing domain.
Chen, H., Qi, X., Faulkner, R.A., Schumacher, M.M., Donnelly, L.M., DeBose-Boyd, R.A., Li, X.(2022) Nat Commun 13: 4273-4273
- PubMed: 35879350
- DOI: https://doi.org/10.1038/s41467-022-32025-5
- Primary Citation of Related Structures:
8DJK, 8DJM - PubMed Abstract:
3-Hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) is the rate-limiting enzyme in cholesterol synthesis and target of cholesterol-lowering statin drugs. Accumulation of sterols in endoplasmic reticulum (ER) membranes accelerates degradation of HMGCR, slowing the synthesis of cholesterol. Degradation of HMGCR is inhibited by its binding to UBIAD1 (UbiA prenyltransferase domain-containing protein-1). This inhibition contributes to statin-induced accumulation of HMGCR, which limits their cholesterol-lowering effects. Here, we report cryo-electron microscopy structures of the HMGCR-UBIAD1 complex, which is maintained by interactions between transmembrane helix (TM) 7 of HMGCR and TMs 2-4 of UBIAD1. Disrupting this interface by mutagenesis prevents complex formation, enhancing HMGCR degradation. TMs 2-6 of HMGCR contain a 170-amino acid sterol sensing domain (SSD), which exists in two conformations-one of which is essential for degradation. Thus, our data supports a model that rearrangement of the TMs in the SSD permits recruitment of proteins that initate HMGCR degradation, a key reaction in the regulatory system that governs cholesterol synthesis.
- Department of Molecular Genetics, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Organizational Affiliation: