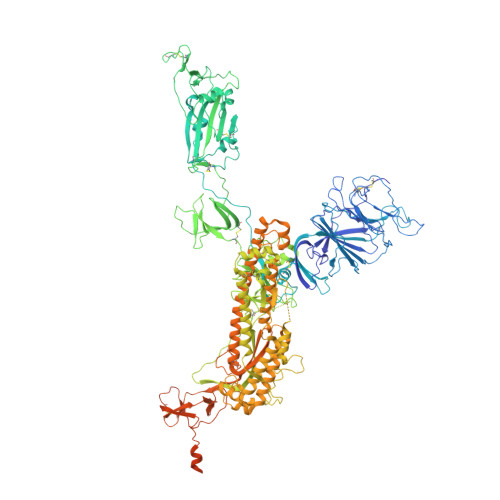
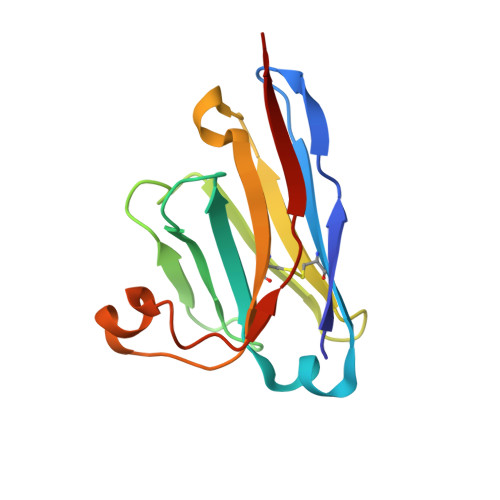
Superimmunity by pan-sarbecovirus nanobodies.
Xiang, Y., Huang, W., Liu, H., Sang, Z., Nambulli, S., Tubiana, J., Williams Jr., K.L., Duprex, W.P., Schneidman-Duhovny, D., Wilson, I.A., Taylor, D.J., Shi, Y.(2022) Cell Rep 39: 111004-111004
- PubMed: 35738279
- DOI: https://doi.org/10.1016/j.celrep.2022.111004
- Primary Citation of Related Structures:
8CWU, 8CWV, 8CXN, 8CXQ, 8CY6, 8CY7, 8CY9, 8CYA, 8CYB, 8CYC, 8CYD, 8CYJ - PubMed Abstract:
Vaccine boosters and infection can facilitate the development of SARS-CoV-2 antibodies with improved potency and breadth. Here, we observe superimmunity in a camelid extensively immunized with the SARS-CoV-2 receptor-binding domain (RBD). We rapidly isolate a large repertoire of specific ultra-high-affinity nanobodies that bind strongly to all known sarbecovirus clades using integrative proteomics. These pan-sarbecovirus nanobodies (psNbs) are highly effective against SARS-CoV and SARS-CoV-2 variants, including Omicron, with the best median neutralization potency at single-digit nanograms per milliliter. A highly potent, inhalable, and bispecific psNb (PiN-31) is also developed. Structural determinations of 13 psNbs with the SARS-CoV-2 spike or RBD reveal five epitope classes, providing insights into the mechanisms and evolution of their broad activities. The highly evolved psNbs target small, flat, and flexible epitopes that contain over 75% of conserved RBD surface residues. Their potencies are strongly and negatively correlated with the distance of the epitopes from the receptor binding sites.
- Department of Cell Biology, University of Pittsburgh, Pittsburgh, PA 15213, USA; Department of Pharmacological Sciences, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Organizational Affiliation: