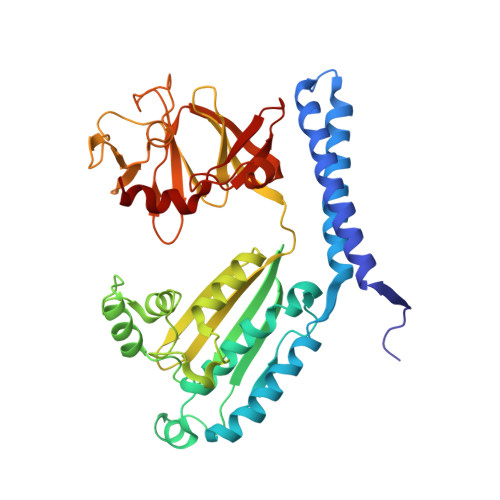
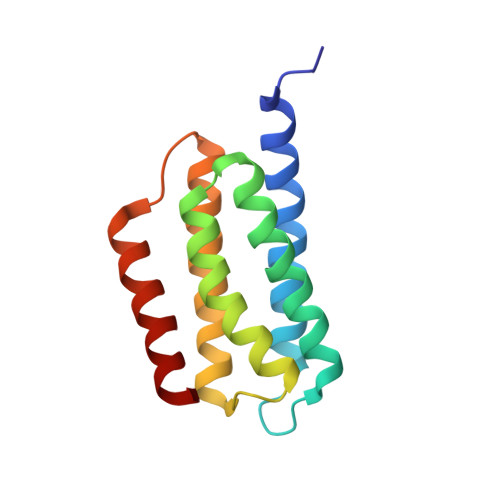
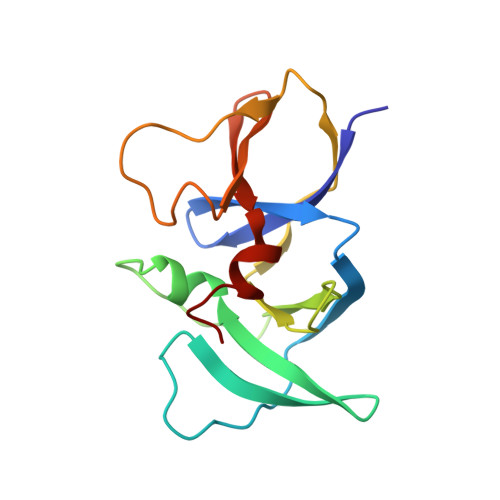
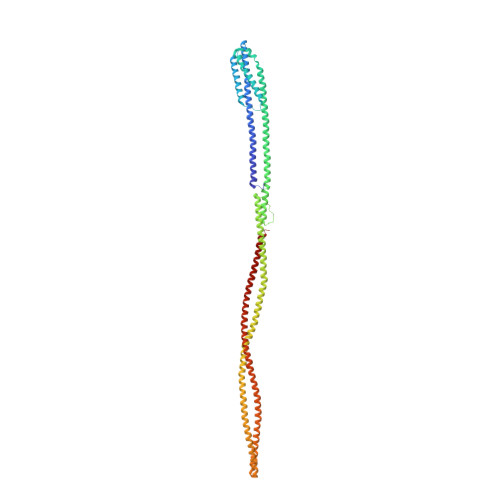
Structure of the native chemotaxis core signaling unit from phage E-protein lysed E. coli cells.
Cassidy, C.K., Qin, Z., Frosio, T., Gosink, K., Yang, Z., Sansom, M.S.P., Stansfeld, P.J., Parkinson, J.S., Zhang, P.(2023) mBio 14: e0079323-e0079323
- PubMed: 37772839
- DOI: https://doi.org/10.1128/mbio.00793-23
- Primary Citation of Related Structures:
8C5V - PubMed Abstract:
Bacterial chemotaxis is a ubiquitous behavior that enables cell movement toward or away from specific chemicals. It serves as an important model for understanding cell sensory signal transduction and motility. Characterization of the molecular mechanisms underlying chemotaxis is of fundamental interest and requires a high-resolution structural picture of the sensing machinery, the chemosensory array. In this study, we combine cryo-electron tomography and molecular simulation to present the complete structure of the core signaling unit, the basic building block of chemosensory arrays, from Escherichia coli . Our results provide new insight into previously poorly-resolved regions of the complex and offer a structural basis for designing new experiments to test mechanistic hypotheses.
- Diamond Light Source , Didcot, United Kingdom.
Organizational Affiliation: