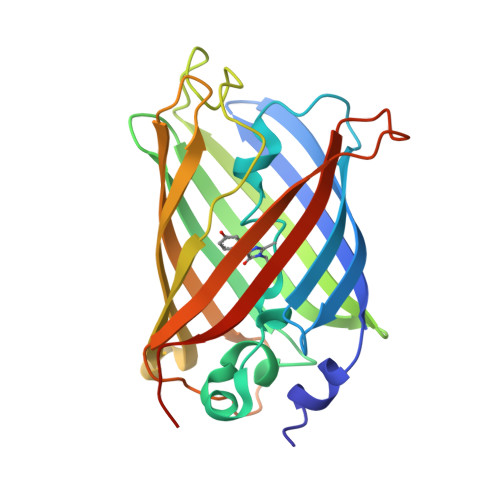
Crystal Structure of Bright Fluorescent Protein BrUSLEE with Subnanosecond Fluorescence Lifetime; Electric and Dynamic Properties.
Goryacheva, E., Efremov, R., Krylov, N., Artemyev, I., Bogdanov, A., Mamontova, A., Pletnev, S., Pletneva, N., Pletnev, V.(2023) Int J Mol Sci 24
- PubMed: 37047378
- DOI: https://doi.org/10.3390/ijms24076403
- Primary Citation of Related Structures:
8BVG - PubMed Abstract:
The rapid development of new microscopy techniques for cell biology has exposed the need for genetically encoded fluorescent tags with special properties. Fluorescent biomarkers of the same color and spectral range and different fluorescent lifetimes (FLs) became useful for fluorescent lifetime image microscopy (FLIM). One such tag, the green fluorescent protein BrUSLEE (Bright Ultimately Short Lifetime Enhanced Emitter), having an extremely short subnanosecond component of fluorescence lifetime (FL~0.66 ns) and exceptional fluorescence brightness, was designed for FLIM experiments. Here, we present the X-ray structure and discuss the structure-functional relations of BrUSLEE. Its development from the EGFP (enhanced green fluorescent proteins) precursor (FL~2.83 ns) resulted in a change of the chromophore microenvironment due to a significant alteration in the side chain conformations. To get further insight into molecular details explaining the observed differences in the photophysical properties of these proteins, we studied their structural, dynamic, and electric properties by all-atom molecular-dynamics simulations in an aqueous solution. It has been shown that compared to BrUSLEE, the mobility of the chromophore in the EGFP is noticeably limited by nonbonded interactions (mainly H-bonds) with the neighboring residues.
- Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Ulitsa Miklukho-Maklaya, 16/10, 117997 Moscow, Russia.
Organizational Affiliation: