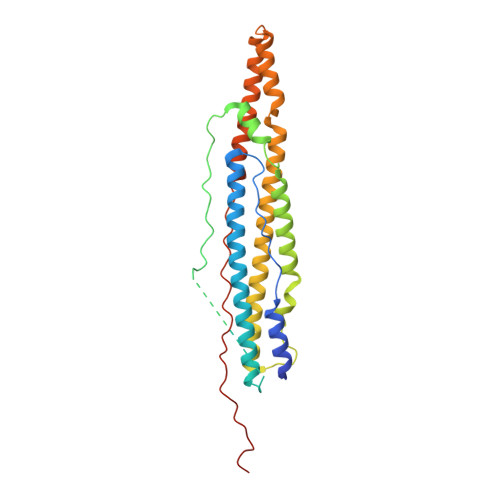
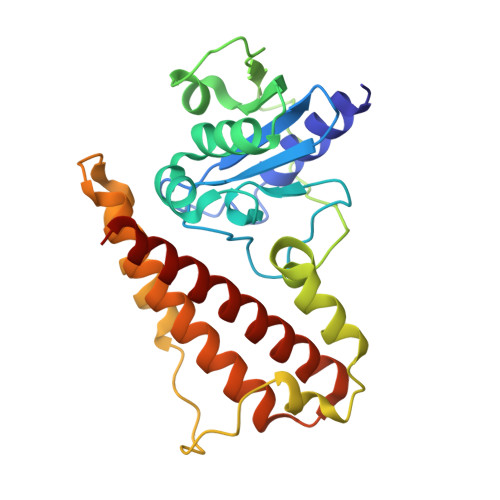
The crystal structure of the EspB-EspK virulence factor-chaperone complex suggests an additional type VII secretion mechanism in Mycobacterium tuberculosis.
Gijsbers, A., Eymery, M., Gao, Y., Menart, I., Vinciauskaite, V., Siliqi, D., Peters, P.J., McCarthy, A., Ravelli, R.B.G.(2022) J Biological Chem 299: 102761-102761
- PubMed: 36463964
- DOI: https://doi.org/10.1016/j.jbc.2022.102761
- Primary Citation of Related Structures:
8AKO - PubMed Abstract:
Pathogenic species from the Mycobacterium genus are responsible for a number of adverse health conditions in humans and animals that threaten health security and the economy worldwide. Mycobacteria have up to five specialized secretion systems (ESX-1 to ESX-5) that transport virulence factors across their complex cell envelope to facilitate manipulation of their environment. In pathogenic species, these virulence factors influence the immune system's response and are responsible for membrane disruption and contributing to cell death. While structural details of these secretion systems have been recently described, gaps still remain in the structural understanding of the secretion mechanisms of most substrates. Here, we describe the crystal structure of Mycobacterium tuberculosis ESX-1 secretion-associated substrate EspB bound to its chaperone EspK. We found that EspB interacts with the C-terminal domain of EspK through its helical tip. Furthermore, cryogenic electron microscopy, size exclusion chromatography analysis, and small-angle X-ray scattering experiments show that EspK keeps EspB in its secretion-competent monomeric form and prevents its oligomerization. The structure presented in this study suggests an additional secretion mechanism in ESX-1, analogous to the chaperoning of proline-glutamate (PE)-proline-proline-glutamate (PPE) proteins by EspG, where EspK facilitates the secretion of EspB in Mycobacterium species.
- Division of Nanoscopy, Maastricht Multimodal Molecular Imaging Institute (M4i), Maastricht University, Maastricht, the Netherlands.
Organizational Affiliation: