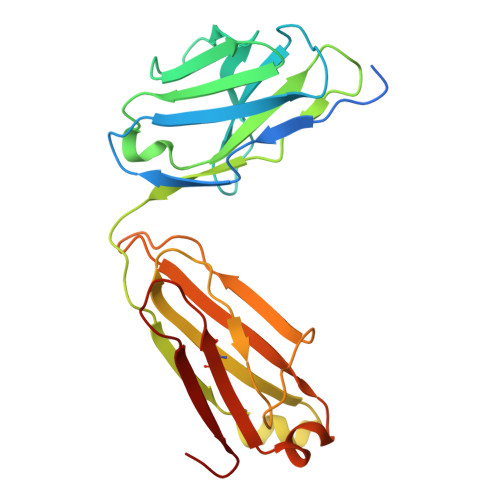
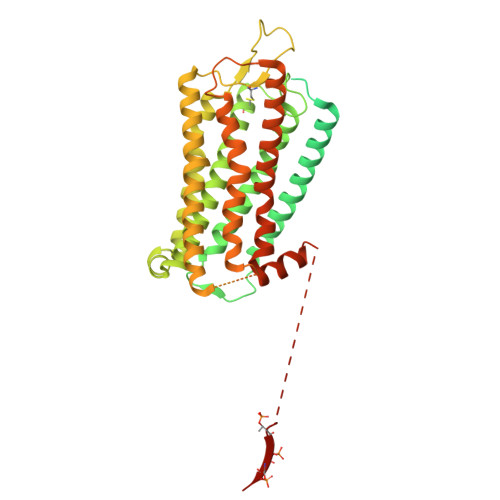
Molecular mechanism of the arrestin-biased agonism of neurotensin receptor 1 by an intracellular allosteric modulator.
Sun, D., Li, X., Yuan, Q., Wang, Y., Shi, P., Zhang, H., Wang, T., Sun, W., Ling, S., Liu, Y., Lai, J., Xie, W., Yin, W., Liu, L., Xu, H.E., Tian, C.(2025) Cell Res 35: 284-295
- PubMed: 40118988
- DOI: https://doi.org/10.1038/s41422-025-01095-7
- Primary Citation of Related Structures:
8ZYT, 8ZYU, 8ZYY, 9M0D - PubMed Abstract:
Biased allosteric modulators (BAMs) of G protein-coupled receptors (GPCRs) have been at the forefront of drug discovery owing to their potential to selectively stimulate therapeutically relevant signaling and avoid on-target side effects. Although structures of GPCRs in complex with G protein or GRK in a BAM-bound state have recently been resolved, revealing that BAM can induce biased signaling by directly modulating interactions between GPCRs and these two transducers, no BAM-bound GPCR-arrestin complex structure has yet been determined, limiting our understanding of the full pharmacological profile of BAMs. Herein, we developed a chemical protein synthesis strategy to generate neurotensin receptor 1 (NTSR1) with defined hexa-phosphorylation at its C-terminus and resolved high-resolution cryo-EM structures (2.65-2.88 Å) of NTSR1 in complex with both β-arrestin1 and the BAM SBI-553. These structures revealed a unique "loop engagement" configuration of β-arrestin1 coupling to NTSR1 in the presence of SBI-553, markedly different from the typical "core engagement" configuration observed in the absence of BAMs. This configuration is characterized by the engagement of the intracellular loop 3 of NTSR1 with a cavity in the central crest of β-arrestin1, representing a previously unobserved, arrestin-selective conformation of GPCR. Our findings fill the critical knowledge gap regarding the regulation of GPCR-arrestin interactions and biased signaling by BAMs, which would advance the development of safer and more efficacious GPCR-targeted therapeutics.
- Hefei National Laboratory for Physical Sciences at the Microscale, Joint Center for Biological Analytical Chemistry, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, China.
Organizational Affiliation: