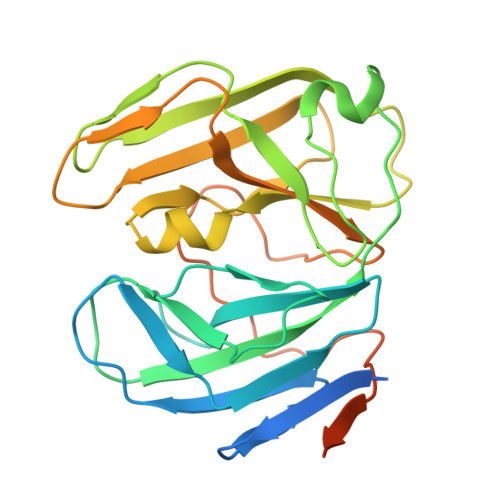
Structural definition of pseudorabies virus dUTPase reveals a novel folding dimer in the herpesvirus family.
Li, J., Ma, S., Yang, R., Xu, J., Wang, Y., Ye, S.(2024) Int J Biol Macromol 280: 135696-135696
- PubMed: 39284464
- DOI: https://doi.org/10.1016/j.ijbiomac.2024.135696
- Primary Citation of Related Structures:
8ZWQ - PubMed Abstract:
The pseudorabies virus (PRV) causes severe and fatal acute respiratory disease in pigs. During PRV proliferation, the enzyme deoxyuridine 5'-triphosphate nucleotide hydrolase (dUTPase) plays a pivotal role in maintaining a low dUTP/dTTP ratio, thereby ensuring the accuracy of viral DNA replication. However, its structure and catalytic mechanisms have not been fully elucidated. Here, we report the crystal structure of PRV dUTPase at a 2.24 Å resolution and demonstrate an unprecedented dimeric architecture, with a conserved enzyme activity center of the herpesvirus family. The enzyme activity center is located in a cavity between the two domains, forming a pocket for binding substrate dUMP and magnesium ions. Remarkably, the exquisite interface of the dimer is primarily composed of four antiparallel β-sheets, which form 11 hydrogen bonds between the residues P33-V36 and R242-A248 to maintain protein stability. To the best of our knowledge, this is the first report demonstrating that dUTPase exists as a dimer in the herpesvirus family. These findings not only present a novel fold dimeric structure but also deepen the scope of our comprehension of structural diversity in dUTPase family.
- Frontiers Science Center for Synthetic Biology (Ministry of Education), Tianjin Key Laboratory of Function and Application of Biological Macromolecular Structures, School of Life Sciences, Tianjin University, Tianjin, China.
Organizational Affiliation: