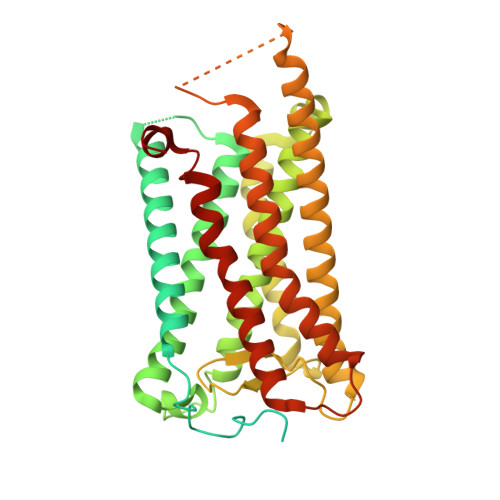
Molecular basis for ligand recognition and receptor activation of the prostaglandin D2 receptor DP1.
Xu, J., Wu, Y., Xu, Y., Li, Y., He, X., Zhang, H., Wang, J.J., Hou, J., Li, J., Hu, W., Wu, K., Yuan, Q., Wu, C., Xu, H.E.(2025) Proc Natl Acad Sci U S A 122: e2501902122-e2501902122
- PubMed: 40440061
- DOI: https://doi.org/10.1073/pnas.2501902122
- Primary Citation of Related Structures:
8ZVZ, 8ZW0, 9UWD - PubMed Abstract:
The prostaglandin D2 receptor 1 (DP1), a rhodopsin-like Class A GPCR, orchestrates critical physiological and pathological processes, ranging from sleep regulation to inflammatory responses and cardiovascular function. Despite its therapeutic significance, structural insights into DP1 activation mechanisms have remained elusive. Here, using cryoelectron microscopy (cryo-EM), we determined high-resolution structures of human DP1 in both inactive and active states, with the latter captured in complex with its endogenous agonist PGD2 or the synthetic agonist BW245C, bound to the stimulatory G protein, Gs. Our structures, coupled with functional and mutagenesis studies, unveiled unique structural features of DP1, including an alternative activation mechanism, ligand-selectivity determinants, and G protein coupling characteristics. These molecular insights provide a rational framework for designing selective DP1-targeted therapeutics, both agonists and antagonists, with enhanced specificity and reduced off-target effects, opening broad avenues for treating DP1-associated disorders.
- State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Organizational Affiliation: