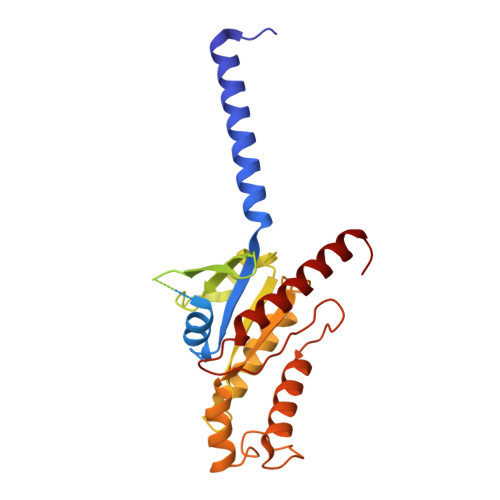
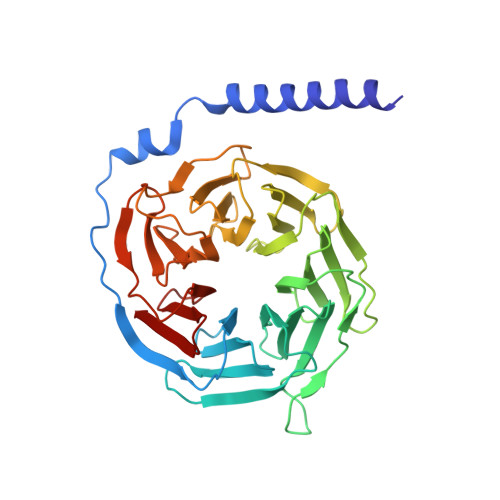
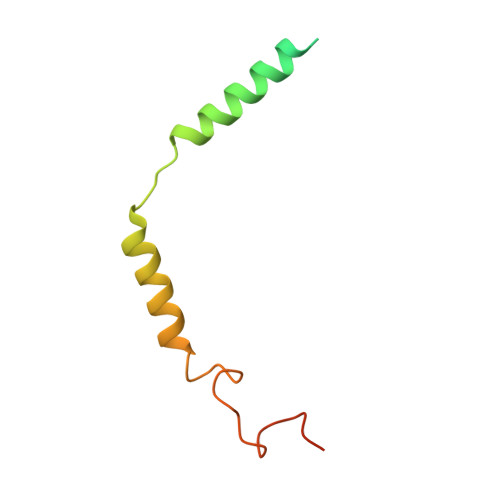
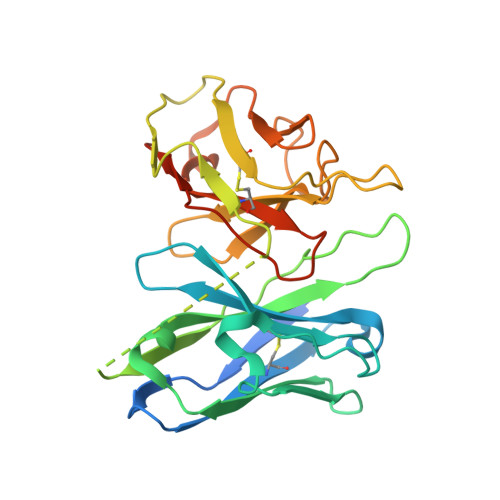
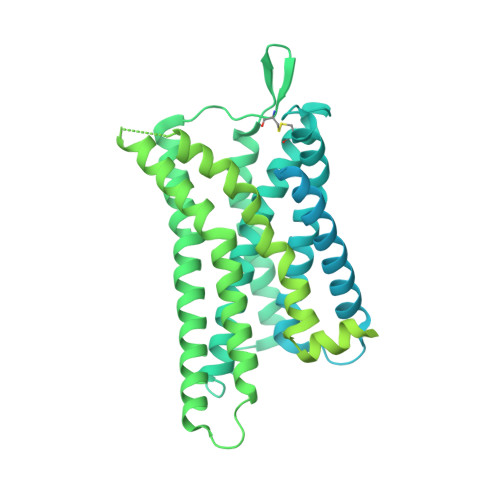
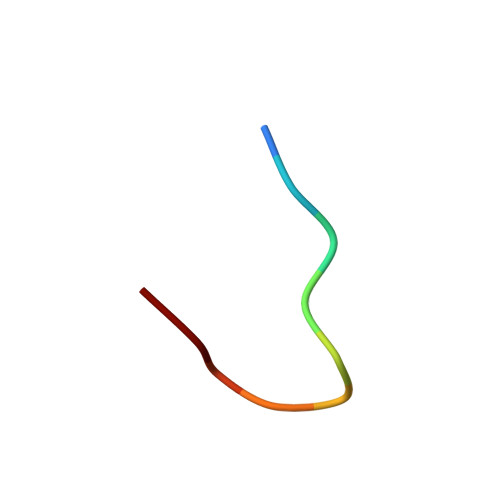
Molecular recognition of the atypical chemokine-like peptide GPR15L by its cognate receptor GPR15.
Zhang, Z., Zheng, Y., Xu, L., Yue, Y., Xu, K., Li, F., Xu, F.(2024) Cell Discov 10: 69-69
- PubMed: 38918398
- DOI: https://doi.org/10.1038/s41421-024-00698-5
- Primary Citation of Related Structures:
8ZQE - iHuman Institute, ShanghaiTech University, Shanghai, China.
Organizational Affiliation: