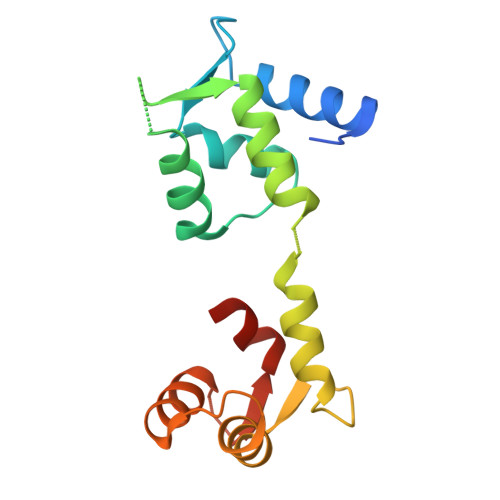
Structural insights into the dual Ca 2+ -sensor-mediated activation of the PPEF phosphatase family.
Liu, J., Wu, C., Liu, Y., Chen, Q., Ding, Y., Lin, Z., Pan, L., Xiao, K., Li, J., Liu, Z., Liu, W.(2025) Nat Commun 16: 3120-3120
- PubMed: 40169586
- DOI: https://doi.org/10.1038/s41467-025-58261-z
- Primary Citation of Related Structures:
8ZLW, 8ZLX - PubMed Abstract:
Serine/threonine-protein phosphatases with EF-hands (PPEFs) are a family of highly conserved proteins implicated in cancer and neuronal degeneration. The initially characterized member, Drosophila melanogaster retinal degeneration C (RDGC) contains a calmodulin (CaM)-interacting extended-IQ motif and a Ca 2+ -binding EF-like/EF-hand tandem. However, the molecular regulation of PPEF is poorly understood. In this study, we use cryogenic-electron microscopy to delineate the structures of the RDGC/CaM holoenzyme. In the absence of Ca 2+ , CaM and the EF-like/EF-hand tandem allow the extended-IQ motif to block substrate access to the catalytic sites, constituting an auto-inhibitory mechanism. Upon Ca 2+ binding, CaM and the EF-like/EF-hand tandem drive drastic conformational changes in the extended-IQ motif to unlock the catalytic sites. This dual Ca 2+ -sensor-mediated activation is evolutionarily conserved in mammals. This study provides mechanistic insight into the molecular activation of PPEFs, paving the way for the development of therapeutic strategies for PPEF-related human diseases.
- Shenzhen Key Laboratory for Neuronal Structural Biology, Biomedical Research Institute, Shenzhen Peking University-The Hong Kong University of Science and Technology Medical Center, Shenzhen, 518036, Guangdong, China.
Organizational Affiliation: