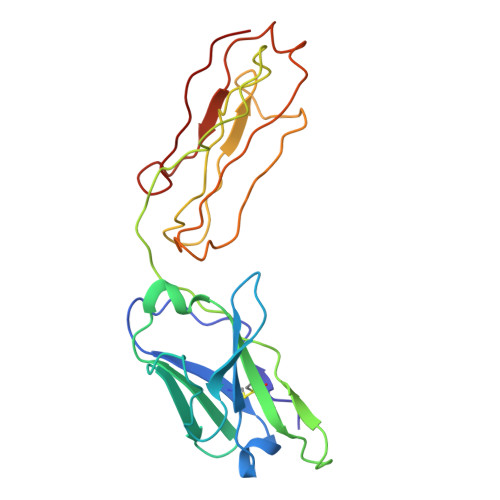
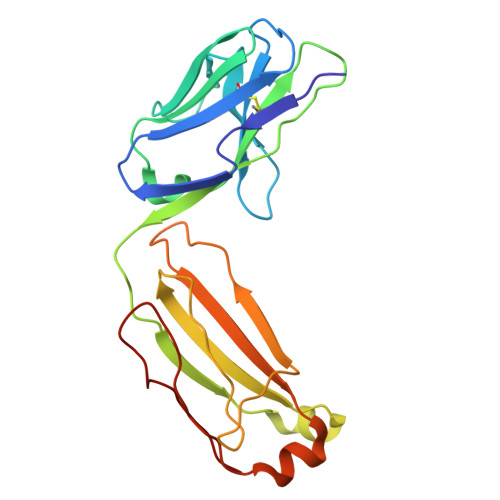
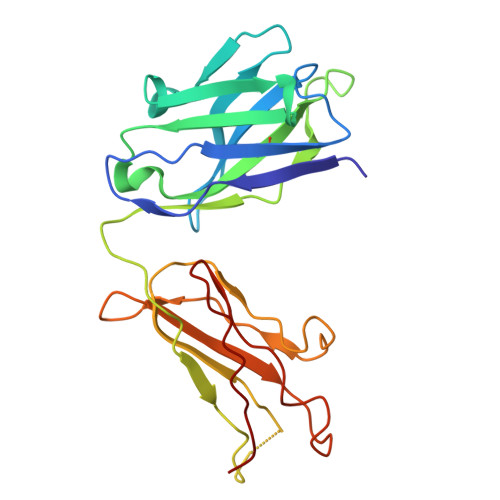
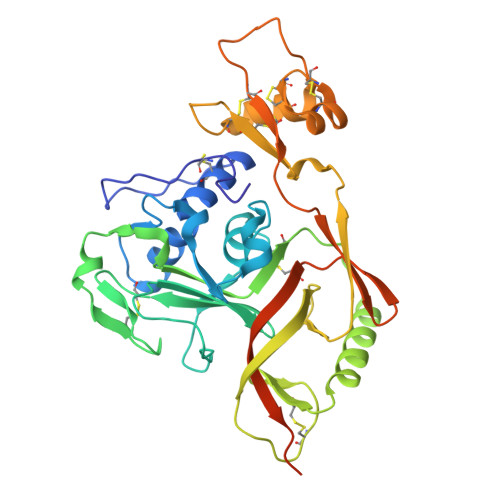
Discovery and characterization of potent broadly neutralizing antibodies from human survivors of severe fever with thrombocytopenia syndrome.
Zhang, S., Shang, H., Han, S., Li, J., Peng, X., Wu, Y., Yang, X., Leng, Y., Wang, F., Cui, N., Xu, L., Zhang, H., Guo, Y., Xu, X., Zhang, N., Liu, W., Li, H.(2024) EBioMedicine 111: 105481-105481
- PubMed: 39644769
- DOI: https://doi.org/10.1016/j.ebiom.2024.105481
- Primary Citation of Related Structures:
8ZHQ - PubMed Abstract:
Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging tick-borne phlebovirus that causes viral hemorrhagic fever. Pandemic concerns have arisen due to the increased human-to-human transmission and high mortality rate, highlighting the urgent need for specific therapeutics. Our observational study characterized the memory B cell response to natural SFTSV infection in four survivors. Monoclonal antibodies (mAbs) targeting the SFTSV glycoprotein N (Gn) were isolated and tested for in vitro neutralizing activities and effects on virus binding. Structural analysis was performed to identify neutralizing epitopes recognized by the mAbs. Prophylactical and therapeutical protections were evaluated using a lethal SFTSV infection model. The selected mAbs exhibiting neutralizing activity primarily originate from the IGHV5-51 and IGHV3-30 germlines and target four distinct antigenic sites on SFTSV Gn. These elite mAbs effectively blocked the interaction between Gn and the cell receptor, preventing infections from five phylogenetically distinct SFTSV clades. Structural analysis revealed a novel neutralizing epitope located within SFTSV Gn domain I recognized by the elite mAbs. In mice of lethal infections with different SFTSV strains, administering a low dose of elite mAbs significantly improved survival rates in both prophylactic and therapeutic settings. This study identifies potent broadly neutralizing antibodies that holds promise for use in humans against SFTSV infection and highlights inhibition of receptor binding as a crucial mechanism for effective antibody-mediated neutralization against phleboviruses. The National Key Research and Development Plan of China (2018YFE0200401, 2022YFC2303300), National Natural Science Foundation of China (81825019), China Postdoctoral Science Foundation (2023M741824).
- State Key Laboratory of Pathogen and Biosecurity, Academy of Military Medical Sciences, Beijing, 100071, PR China; College of Biological Science and Food Engineering, Southwest Forestry University, Kunming, 650224, PR China.
Organizational Affiliation: