Study on the function and diversity of key enzymes degrading fucoidan in Kiritimatiellaeota
Zhao, P., Bai, L., Wang, S.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Alpha-L-fucosidase | 848 | Pontiella sulfatireligans | Mutation(s): 0 Gene Names: SCARR_01153 | 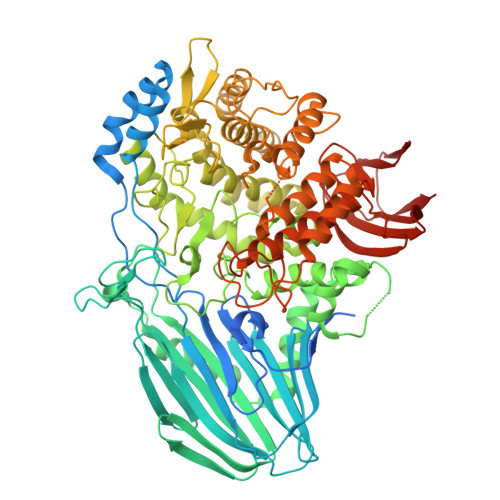 | |
UniProt | |||||
Find proteins for A0A6C2UI12 (Pontiella sulfatireligans) Explore A0A6C2UI12 Go to UniProtKB: A0A6C2UI12 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A6C2UI12 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 105.293 | α = 90 |
| b = 106.204 | β = 91.84 |
| c = 72.598 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| XDS | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |