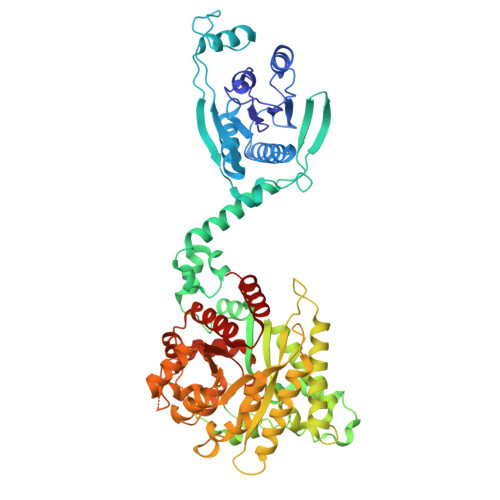
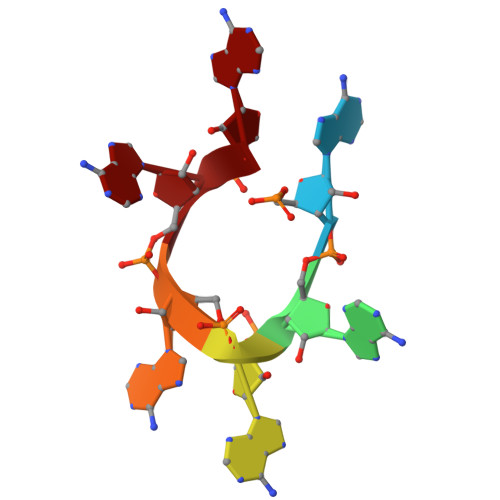
Antiviral signaling of a type III CRISPR-associated deaminase.
Li, Y., Li, Z., Yan, P., Hua, C., Kong, J., Wu, W., Cui, Y., Duan, Y., Li, S., Li, G., Ji, S., Chen, Y., Zhao, Y., Yang, P., Hu, C., Lu, M., Chen, M., Xiao, Y.(2025) Science 387: eadr0393-eadr0393
- PubMed: 39666823
- DOI: https://doi.org/10.1126/science.adr0393
- Primary Citation of Related Structures:
8Z3K, 8Z3P, 8Z3R, 8Z40 - PubMed Abstract:
Prokaryotes have evolved diverse defense strategies against viral infection, such as foreign nucleic acid degradation by CRISPR-Cas systems and DNA/RNA synthesis inhibition via nucleotide pool depletion. Here, we report an antiviral mechanism of type III CRISPR-Cas-regulated ATP depletion, where ATP is converted into ITP by CRISPR-Cas-associated adenosine deaminase (CAAD) upon activation by either cA 4 or cA 6 , followed by hydrolysis into IMP by Nudix hydrolase, ultimately resulting in cell growth arrest. The cryo-electron microscopy structures of CAAD in its apo and activated forms, together with biochemical evidence, revealed how cA 4 /cA 6 binds to the CARF domain and abrogates CAAD autoinhibition, inducing substantial conformational changes that reshape the structure of CAAD and induce its deaminase activity. Our results reveal the mechanism of a CRISPR-Cas-regulated ATP depletion antiviral strategy.
- Department of Pharmacology, School of Pharmacy, China Pharmaceutical University, Nanjing, China.
Organizational Affiliation: