Structural and mechanistic insights into the allosteric activation of p38alpha MAP kinase via specific docking interactions
Zhang, Y.Y., Pei, C.J., He, Q.Q., Luo, Z.P., Wu, J.W., Wang, Z.X.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Mitogen-activated protein kinase 14 | 379 | Mus musculus | Mutation(s): 0 Gene Names: Mapk14, Crk1, Csbp1, Csbp2 EC: 2.7.11.24 | 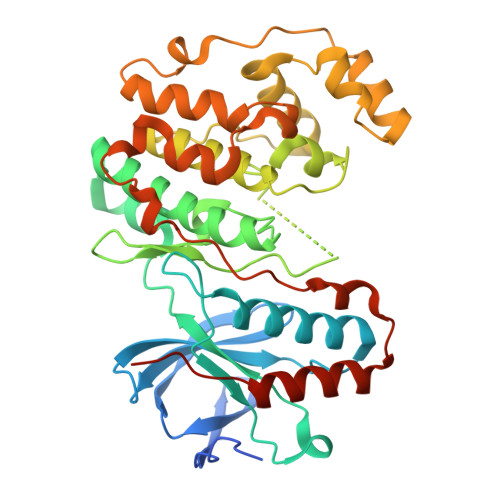 | |
UniProt | |||||
Find proteins for P47811 (Mus musculus) Explore P47811 Go to UniProtKB: P47811 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P47811 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Tyrosine-protein phosphatase non-receptor type 7 | 16 | Homo sapiens | Mutation(s): 1 EC: 3.1.3.48 | 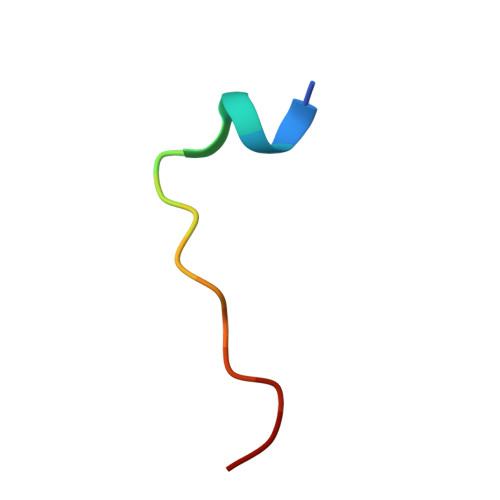 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P35236 (Homo sapiens) Explore P35236 Go to UniProtKB: P35236 | |||||
PHAROS: P35236 GTEx: ENSG00000143851 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P35236 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 81.96 | α = 90 |
| b = 81.96 | β = 90 |
| c = 122.4 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| XDS | data reduction |
| XSCALE | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Ministry of Science and Technology (MoST, China) | China | 2019YFA0802500 |
| Ministry of Science and Technology (MoST, China) | China | 2022YFA0806504 |